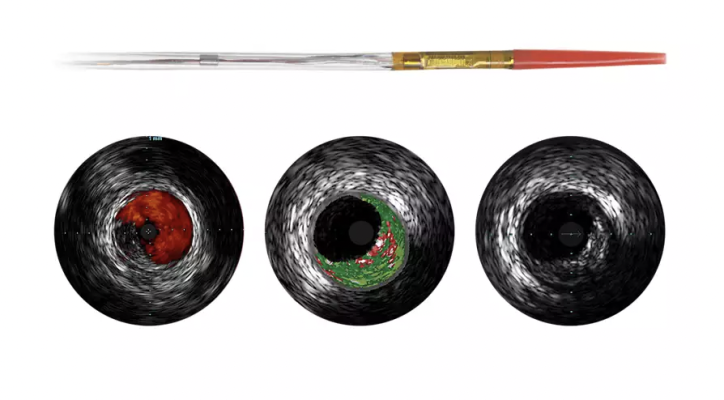
February 16, 2023 — Northeast Scientific Inc., the pioneers in reprocessing single use peripheral vascular catheters, announced this week it has received FDA 510(k) clearance for reprocessing the Philips IVUS Eagle Eye Platinum RX Digital catheter.
CEO and Founder, Craig Allmendinger shared his thoughts on the impact of adding another device to Northeast Scientifics growing product portfolio, “The reprocessed Eagle Eye catheter is another high-cost complex interventional device that will bring considerable savings to Office Based Labs and Hospital Cath Labs nationwide. Having reprocessed multiple sizes of the peripheral IVUS device for the last three years to great success, it was an easy choice to pursue the Eagle Eye version.”
With the clearance of the first ever reprocessed Atherectomy catheter last year, the Philips Turbo Elite Laser Catheter, the company is focused on expanding potential cost savings wherever these procedures are performed through unmatched innovation and commitment to quality and patient safety.
Director of Product Development, Matt Farley concluded with; “As always, it’s a testament to our team, from R&D to Product Development, Quality and Regulatory and the technicians helping do the work on validations. We’ve spent a lot of time honing in how we work through our product pipeline to submit 510(k)s and expect to have multiple submitted in 2023, the Eagle Eye is just the start.”
The company has said that it will have an additional announcement soon regarding when the NES Reprocessed Eagle Eye Platinum RX Digital IVUS Catheter device will be available for sale.
For more information: https://www.smarthealth-care.com/


 January 29, 2026
January 29, 2026