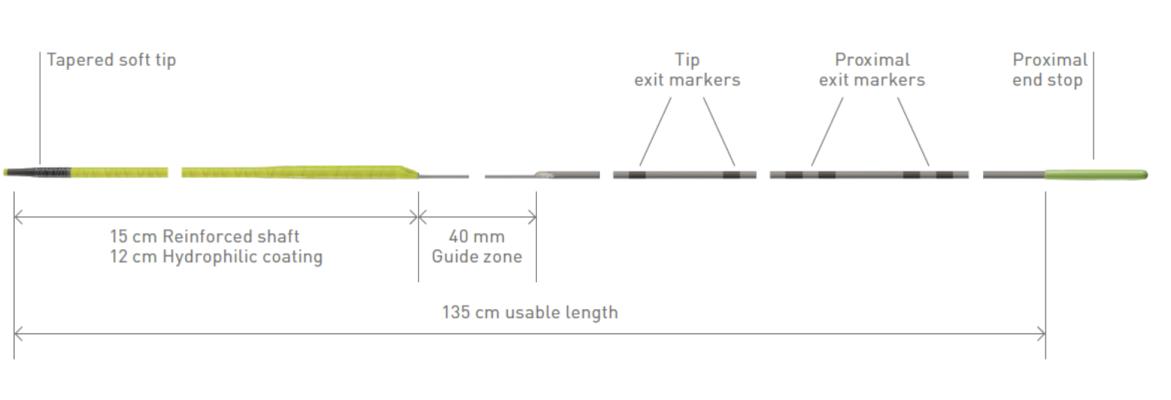
February 9, 2024 — BIOTRONIK introduced the Micro Rx catheter, a novel rapid exchange microcatheter developed to easily enhance guidewire support during percutaneous coronary interventions (PCI). This cutting-edge device, exclusively distributed by BIOTRONIK, is manufactured by IMDS (Interventional Medical Device Solutions). Micro Rx catheter marks the fourth IMDS product BIOTRONIK has brought to the U.S., creating a compelling portfolio of devices which includes NHancer Rx, TrapIT, and ReCross catheters.
Dr. Ashish Pershad, MD, Director of Interventional Cardiology at Chandler Regional Medical Center and Mercy Gilbert Medical Center, one of the first users of the MicroRx catheter, shared his experience. “It provides good support and has a low profile allowing for great tip control for coronary guidewires in complex lesions. Not having to switch out your OTW catheter after you cross a difficult lesion is a huge convenience in an era where 190 cm wires are the norm in most cath labs.”
Acknowledging the intricacies involved in navigating through challenging vascular anatomies, such as tortuous vessels and highly stenosed lesions, the Micro Rx catheter is designed to prioritize user-friendly intervention, eliminating the need for trapping. This approach aims to mitigate the risk of vascular trauma during procedures, subsequently reducing the overall duration of patients' exposure to the catheterization lab environment and associated risks. It has the potential to enable non-chronic total occlusion (CTO) operators to successfully treat more complex anatomies.3,4
“IMDS has invested its expertise and dedication into the development of the Micro Rx catheter, with a specific focus on alleviating challenges in PCI,” said Ryan Walters, President at BIOTRONIK USA. “This has resulted in a state-of-the-art solution, benefiting both physicians and the patients. We are proud to bring this innovation to the U.S. market.”
For more information: www.biotronik.com
References:1 Profile compared to Finecross MG Coronary Micro-Guide Catheter 2 End-stop is 6F Guide Extension Catheter compatible 3 Goel PK, Sahu AK, Kasturi S, et al. Guiding principles for the clinical use and selection of microcatheters in complex interventions. Front. Cardiovasc. Med. 2022;9:724608 doi: 10.3389/fcvm.2022.724608 4 Leibundgut, G. et Al. A Novel Device for Additional Guide Wire Support to Cross Tortuous Anatomy and Tight Lesions - The Micro Rx Rapid Exchange Microcatheter. Journal of Invasive Cardiology. 1-9 |


 June 13, 2024
June 13, 2024 









