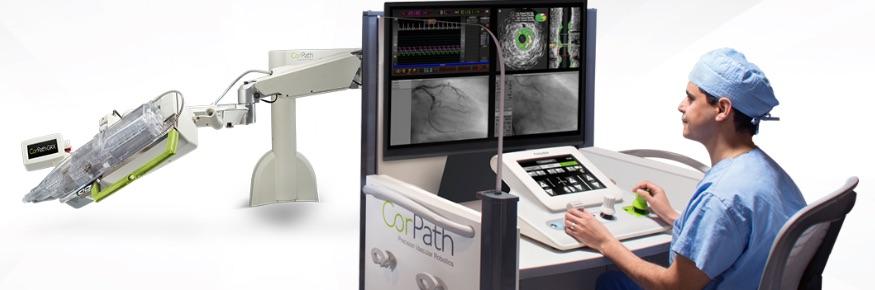
February 20, 2018 — Corindus Vascular Robotics Inc. announced today that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use of its CorPath GRX System in peripheral vascular interventions. The CorPath System is the first and only FDA-cleared medical device, according to the company, to bring robotic precision to both percutaneous coronary intervention (PCI) and peripheral vascular intervention (PVI) procedures.
The CorPath GRX System broadens the capabilities of the CorPath robotic technology platform from exclusively treating coronary artery disease (CAD) to include peripheral artery disease (PAD). It is estimated that 8.5 million people in the United States are living with PAD, a disease of blood vessels outside the heart that commonly affects arteries carrying blood to the lower extremities. In 2017, peripheral procedures were estimated to be a $3.4 billion market, and it is estimated that by 2020, one million PAD procedures will be performed annually in the U.S.1,2
"My colleagues and I have seen first-hand how CorPath GRX can overcome the challenges of manual PCI and I am excited to apply the capabilities of robotics to effectively treat PAD patients," said Alan Lumsden, M.D., chief of the Department of Cardiovascular Surgery at Houston Methodist Hospital. "As a training site for future robotic interventionalists, I look forward to teaching these techniques to further enhance the quality of care for patients with both CAD and PAD."
"CorPath GRX enables me to provide transformational treatment options to my patients suffering from PAD," said Joseph Ricotta, M.D., medical director of vascular surgery and endovascular therapy, Tenet Healthcare, professor of surgery, Charles E Schmidt College of Medicine, FAU. "As a long-time adopter of robotics, I am passionate about the opportunity this technology presents to advance endovascular care while providing a safer work environment for healthcare providers."
For more information: www.corindus.com
References
1. Millennium Research Group 2013 Peripheral Vascular Devices US 2013 Market Analysis RPUS 11PV14 p 98.
2. cdc.gov/PAD.


 November 14, 2025
November 14, 2025 









