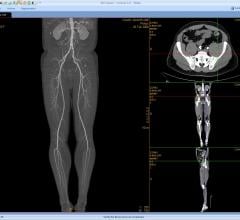
December 8, 2016 — LimFlow SA announced in November that it received the CE Mark for its fully percutaneous LimFlow ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
Shimadzu's latest generation interventional lab angiography imaging system, the Trinias, enables advanced imaging ...
December 7, 2016 — Teleflex Inc. and Vascular Solutions Inc. announced that the companies have entered into a definitive ...
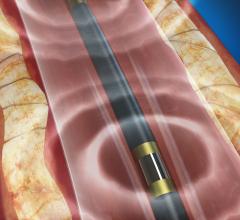
December 6, 2016 — The Spectranetics Corp. announced in late November that its Stellarex 0.014-inch drug-coated ...
A discussion with Todd Brinton, M.D., about the newly FDA-cleared Shockwave Medical Lithoplasty System, at the ...

The volume of information attendees receive at the annual Transcatheter Cardiovascular Therapeutics (TCT) interventional ...
October 24, 2016 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the HawkOne ...
October 20, 2016 — Avinger Inc. recently announced that the company has received expanded indications from the U.S. Food ...
October 12, 2016 — The Society for Vascular Surgery (SVS) has released new reporting standards focused on endovascular ...
October 12, 2016 — Medtronic plc announced recently that the U.S. Food and Drug Administration (FDA) has cleared the ...
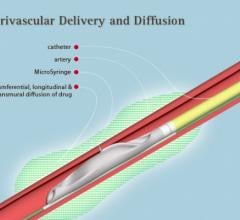
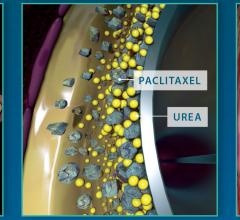
October 7, 2016 — Mercator MedSystems recently announced that 13-month data from the DANCE trial was presented during a ...

September 28, 2016 — The Cardiovascular Research Foundation (CRF) included 11 late-breaking trials and 16 first report ...
September 26, 2016 — C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational ...
September 22, 2016 — New data presented at the Vascular Interventional Advances (VIVA) conference demonstrated the ...
September 19, 2016 — Shockwave Medical announced positive clinical results from the pooled DISRUPT PAD Study, a single ...


 December 08, 2016
December 08, 2016