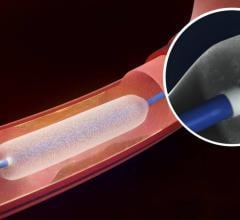
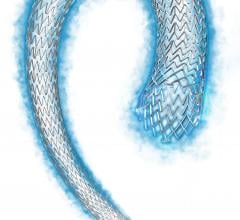
August 20, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Innova ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
The Magellan 10 French Robotic Catheter from Hansen Medical is indicated for use in the peripheral vasculature.
The ...
July 22, 2015 — Cardiovascular Systems Inc. announced that it has received U.S. Food and Drug Administration (FDA) ...
July 17, 2015 - Veryan Medical announced that the first subject has been enrolled in their MIMICS-2 study at ...
July 16, 2015 — Medtronic plc announced that its Protege GPS self-expanding peripheral stent system has received ...

July 8, 2015 - C.R. Bard Inc. announced the publication of results from the LEVANT 2 study in the June 24, 2015, online ...
June 11, 2015 - C.R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has improved the ...
June 10, 2015 - Biotronik announced the publication of promising clinical results regarding its Pulsar-18 peripheral ...
June 10, 2015 - Cordis Corp. announced the launch of the new Elitecross support catheter in the United States and ...
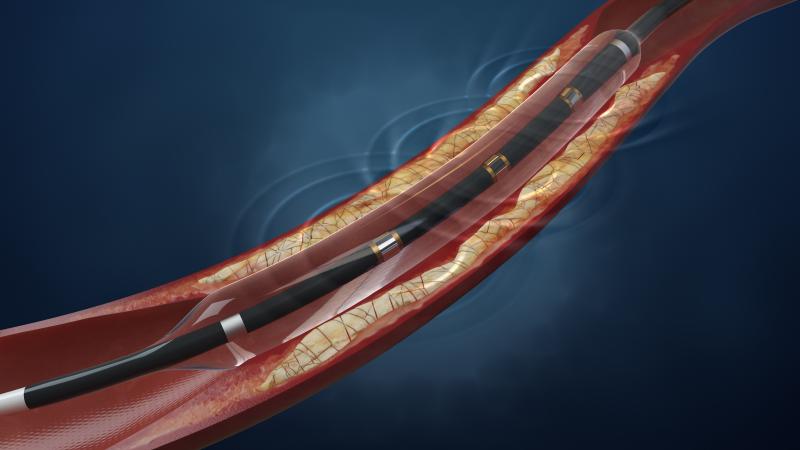
May 27, 2015 — Shockwave Medical announced $40 million in funding, co-led by returning investor Sofinnova Partners and ...
May 22, 2015 — New clinical data from two different studies show the IN.PACT Admiral drug-coated balloon from Medtronic ...
May 6, 2015 — The Spectranetics Corporation announced it is accelerating investments in the Stellarex drug-coated ...
April 29, 2015 — A key trial evaluating the Boston Scientific Eluvia drug-eluting vascular stent system met its primary ...


 August 20, 2015
August 20, 2015