Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
December 31, 2014 — Roxwood Medical Inc. announced U.S. Food and Drug Administration (FDA) clearance for the U.S ...
December 22, 2014 — Advanced Vascular Dynamics (AVD) announced U.S. Food and Drug Administration (FDA) clearance and CE ...
December 17, 2014 — ReFlow Medical Inc. announced U.S. Food and Drug Administration (FDA) clearance for ...
November 20, 2014 — Oxford University researchers presented data that reveal the extent to which smoking causes silent ...
November 17, 2014 — VIVA Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
Nov. 14, 2014 — Covidien releases 12-month results of the DEFINITIVE AR study, the first randomized study designed to ...
November 11, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for ...

Nov. 11, 2014 — Viva Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
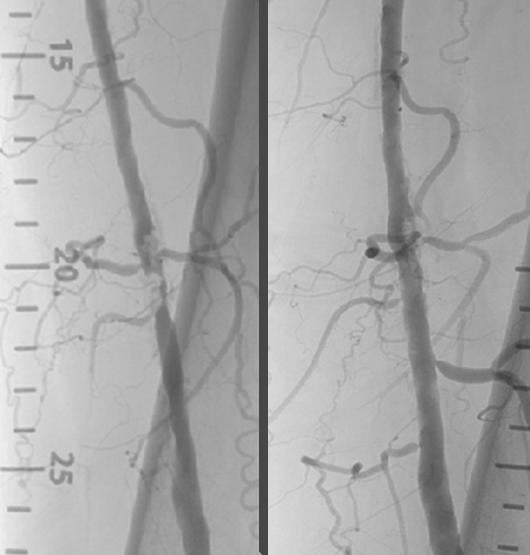
Nov. 11, 2014 — Shockwave Medical announced positive clinical results from Disrupt PAD trial, a single-arm multicenter ...
Nov. 10, 2014 — For the treatment of peripheral artery disease in leg arteries above the knee, the IN.PACT Admiral drug ...
Nov. 7, 2014 — Intact Vascular, a medical device company and developer of products for minimally invasive peripheral ...
Nov. 6, 2014 — Cardiovascular Systems Inc. received the CE Mark for its Stealth 360º Orbital Atherectomy System (OAS) ...
Nov. 5, 2014 — Food and Drug Administration gave 510(k) clearance for the HawkOne directional atherectomy system. The ...
November 4, 2014 — Shockwave Medical announced that the company will present clinical results from DISRUPT PAD, a single ...

 January 05, 2015
January 05, 2015