June 11, 2015 - C.R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has improved the pass-through payment for the Lutonix drug-coated balloon (DCB) under the Medicare hospital outpatient prospective payment system. The purpose of the reimbursement is to cover additional cost to U.S. hospitals for treating Medicare beneficiaries with the Lutonix DCB in the outpatient setting.
After further review, CMS determined that costs associated with DCBs were not included in existing reimbursement for percutaneous transluminal angioplasty, stenting or atherectomy procedures. Therefore, CMS is removing the device offset charge from the calculation and will reimburse the full cost of DCBs in these procedures. This reimbursement determination is retroactive to April 1, 2015.
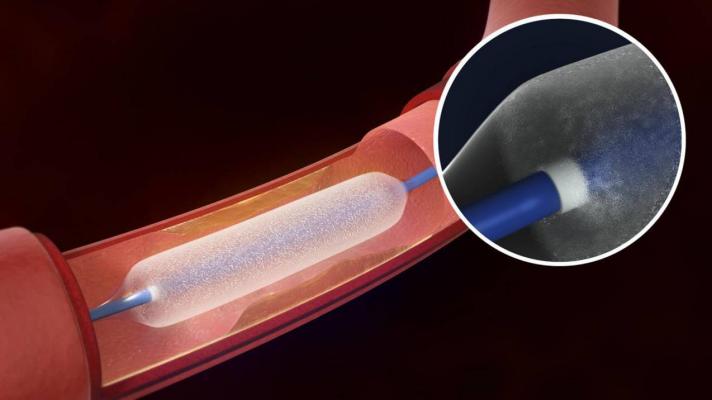
The Lutonix 035 DCB - the first U.S. Food and Drug Administration (FDA)-approved DCB - is an angioplasty balloon coated with a therapeutic dose of the drug paclitaxel. It utilizes standard mechanical dilatation of the vessel to restore blood flow for patients with peripheral arterial disease (PAD) in the femoropopliteal arteries.
For more information: www.crbard.com


 November 14, 2025
November 14, 2025 









