September 16, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance for Shockwave Medical’s ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
This video, provided by Shockwave Medical, demonstrates the Lithoplasty System. It uses ultrasonic waves to treatment ...

Vascular Interventional Advances (VIVA) 2016 released its list late-breaking research presentations in vascular ...
August 24, 2016 — Intact Vascular Inc. announced that the one-year results from its Tack Optimized Balloon Angioplasty ...
August 22, 2016 — Avinger Inc. recently announced the closing of its previously announced public offering of 9,857,800 ...

Due to poor outcomes from percutaneous transluminal angioplasty (PTA) ballooning of vessels alone, or of stenting in ...
August 16, 2016 — Cardiovascular Systems Inc. (CSI) released procedural and 30-day results from its LIBERTY 360° study ...
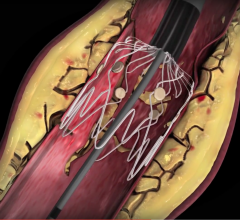
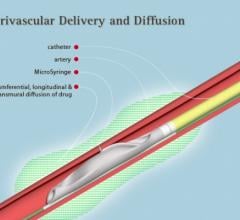
August 10, 2016 — Mercator MedSystems Inc. announced the enrollment of the first critical limb ischemia (CLI) patients ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology ...
August 2, 2016 —The U.S. Food and Drug Administration (FDA) granted market clearance for W. L. Gore & Associates’ Gore ...
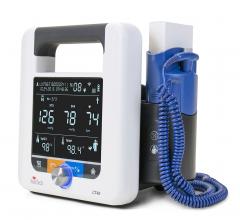
July 18, 2016 — Blood pressure technology company SunTech Medical received U.S. Food and Drug Administration (FDA) ...
July 13, 2016 — VentureMed Group Ltd., specializing in devices for the endovascular treatment of peripheral arterial ...
June 7, 2016 — Intact Vascular Inc. announced that its Tack Optimized Balloon Angioplasty III (TOBA III) clinical trial ...
June 3, 2016 — Cardionovum GmbH recently announced the completion of enrollment of the RAPID trial. Results will be used ...
May 5, 2016 — Intact Vascular Inc. announced that positive twelve-month results from its Tack Optimized Balloon ...

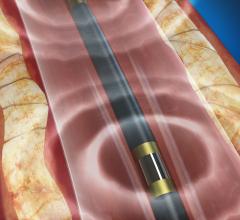
 September 16, 2016
September 16, 2016