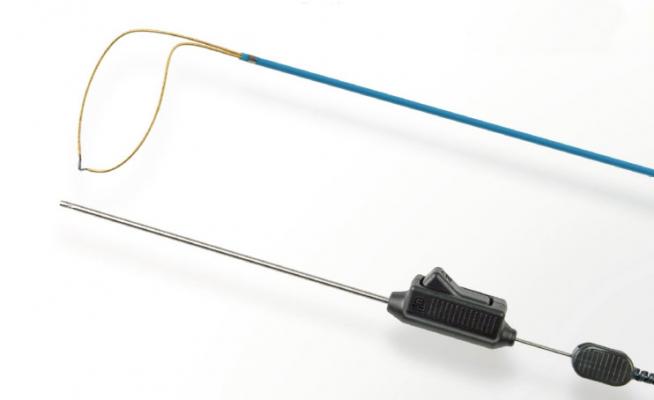
February 7, 2018 — Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S. commercial launch of new versions of its Expro Elite and Sympro Elite Snares for manipulating interventional devices in peripheral procedures.
Both the Expro Elite and Sympro Elite Snares represent next-generation versions of the 0.035-inch interventional snares originally launched in the U.S. in 2008 by Vascular Solutions, now a wholly-owned subsidiary of Teleflex Inc.
Stewart Strong, president and general manager of the Interventional business unit of Teleflex, said both snare products feature:
- A durable nitinol construction that retains its shape and adds strength;
- A radiopaque gold-plated tungsten coil and tip for enhanced visibility;
- 1:1 torque response for controlled positioning and better access to distal targets; and
- A unique locking handle that facilitates secure capture.
The Expro Elite Snare uses a helical loop for capture in all directions. The Sympro Elite Snare uses a 90-degree loop that remains coaxial to the vessel lumen for easy capture. Both snare products feature a preassembled, one-piece design that allows rapid deployment through any 0.035-inch compatible lumen. Both the Expro Elite and Sympro Elite Snares are available in 5, 10, 15, 25 and 35 mm loop diameters for clinical versatility. Both snares are 150 cm in length and are packaged one per box.
Both snares are intended for use in the cardiovascular system and hollow viscus to retrieve and/or manipulate objects using minimally-invasive surgical procedures. Manipulation procedures include retrieval and/or repositioning of intravascular foreign objects such as coils, balloons, catheters, and/or guidewires within the cardiovascular system. The devices are not intended for use in the coronary arteries or neurovasculature.
For more information: www.teleflex.com


 November 14, 2025
November 14, 2025 









