February 12, 2019 — A study presented at the 2018 annual meeting of the Cardiovascular and Interventional Radiology Society of Europe (CIRSE) explored the safety and efficacy of the JETI-8 Peripheral Thrombectomy System for the treatment of acute deep vein thrombosis (DVT). The study, presented by Jean Cournoyer-Rodrigue, M.D., CHUM Research Center, Montreal, showed 83 percent technical success.
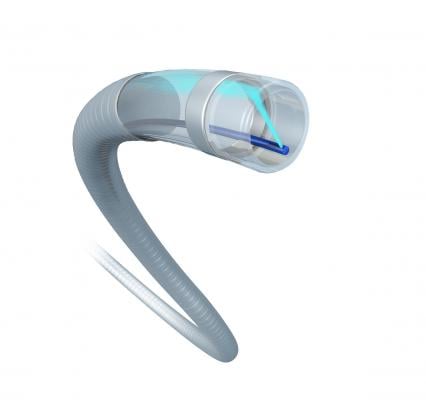
The JETi Thrombectomy System is a device that breaks-up and removes thrombus from the coronary and peripheral vasculature, incorporating a powerful internal jet technology that macerates thrombus just inside its mouth and lubricates the proximally flowing aspirate. The device has a desirable safety profile, allows for single-session treatment potential with or without lytics, has U.S. Food and Drug Administration (FDA) 510K clearance and CE Mark.
Jeti was tested in 23 venous thromboembolism (VTE) procedures, resulting in 83 percent technical success, defined as restoration of integrate flow with the elimination of any obstructing lesion, without overnight CDT. In addition, 4 out of 23 procedures required post-procedure lytic infusion before achieving procedural and technical success, with overnight CDT. Current thrombectomy market leaders such as Angiojet have only demonstrated single-session success of 34 percent in their seminal Pearl registry, according to Walk Vascular. No complication, hemolysis or blood transfusion was reported. Mean thrombus removal across all patients was 93 percent.
For more information: www.cirse.org


 January 05, 2026
January 05, 2026 









