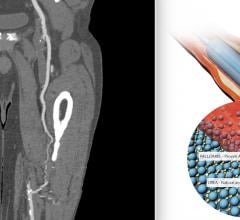
October 20, 2021 — 18-month results from the PRESTIGE* Below-the-Knee (BTK) study have been presented as a late-breaking ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
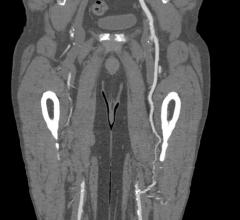
October 20, 2021 — Boston Scientific Corporation announced positive data for the Eluvia Drug-Eluting Vascular Stent ...
Here are the top 10 takeaways from the late-breaking studies on cardiovascular technologies presented at the 2021 Americ ...
June 7, 2021 — A couple years ago a study showed a mortality safety signal in patients who underwent peripheral artery ...
May 17, 2021 — The anticoagulant rivaroxaban (Xarelto), in addition to low-dose aspirin, significantly reduced the ...
February 22, 2021 — The U.S. Food and Drug Administration (FDA) recently cleared the Cook Medical Zilver Vena Venous ...
February 3, 2021 — Modern Vascular is a medical group that has 13 outpatient cath lab clinics to treat peripheral artery ...
January 26, 2021 — Pedra Technology, a privately held company, announced today that the U.S. Food and Drug ...
December 16, 2020 — Efemoral Medical announced the first-in-human (FIH) use of the its Efemoral bioresorbable vascular ...
November 13, 2020 — The new U.S. Food and Drug Administration (FDA) cleared XO Score system was successfully used to ...
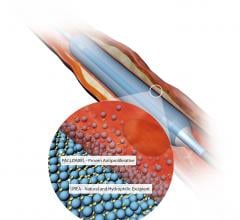
November 3, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Boston Scientific Ranger Drug-coated ...
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...

October 18, 2020 – A large subgroup analysis of a randomized clinical trial showed neither a mortality risk nor benefit ...
October 18, 2020 – The first results from the IN.PACT Below the Knee (BTK) Study, a feasibility study assessing the ...
September 25, 2020 — Philips Healthcare launched its QuickClear mechanical thrombectomy system. The compact, single-use ...


 October 20, 2021
October 20, 2021