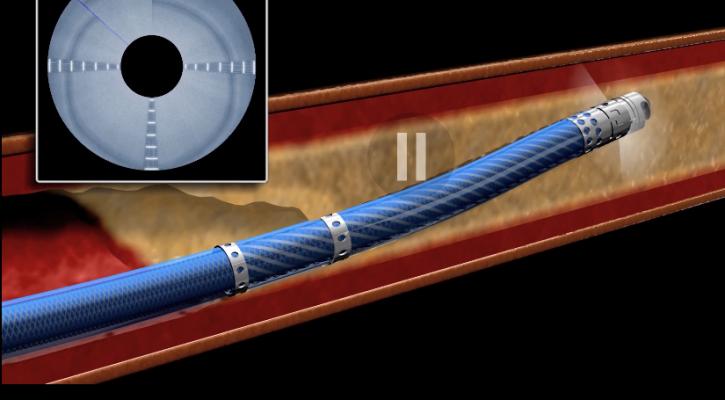
September 14, 2020 — Avinger received U.S. Food and Drug Administration (FDA) 510(k) clearance from the FDA for its Ocelaris next generation image-guided chronic total occlusion (CTO) crossing system for patients with peripheral artery disease (PAD). The new device will be marketed under the brand name TigerEye to reinforce its highly differentiated benefit of providing real-time optical coherence tomography (OCT) imaging from inside the vessel during a CTO-crossing procedure.
TigerEye is a product line extension of Avinger’s Ocelot family of image-guided CTO crossing catheters. Its design elements include an upgrade of the image capture rate to provide high definition, real-time intravascular imaging similar to the company’s Pantheris image-guided atherectomy system and a user-controlled deflectable tip designed to assist in steerability within the lumen. TigerEye also features a new distal tip OCT imaging configuration with faster rotational speeds up to 1,000 RPM designed to penetrate challenging lesions. The TigerEye catheter has a working length of 140 cm and 5 French sheath compatibility for treatment of lesions in the peripheral vessels both above and below the knee.
The company expects to launch of TigerEye in the U.S. in the fourth quarter of 2020.
“I believe that TigerEye represents a major advancement for patients with chronic total occlusions, which presents one of the most significant technical challenges to physicians treating peripheral artery disease. By combining real-time intravascular imaging and the ability to precisely control the device within the vessel, TigerEye provides an important new tool to help interventionalists stay within the true lumen while successfully crossing these challenging lesions," said Jaafer Golzar, Avinger’s chief medical officer. "Intraluminal crossing provides for a wider variety of treatment options following crossing of the CTO and results in less potential for vascular injury, which has been shown to improve long-term clinical outcomes for patients.”
Avinger’s proprietary Lumivascular technology allows physicians, for the first time ever, to see from inside the artery during an atherectomy or CTO crossing procedure by using an imaging modality called optical coherence tomography, or OCT, that is displayed on Avinger’s Lightbox console. Physicians performing atherectomy or crossing CTOs with other devices must rely solely on X-ray as well as tactile feedback to guide their interventions while treating complicated arterial disease. With the Lumivascular approach, physicians can more accurately navigate their devices and treat PAD lesions, thanks to the real-time OCT images generated from inside the artery, without exposing healthcare workers and patients to the negative effects of ionizing radiation.
For more information: www.avinger.com


 November 08, 2024
November 08, 2024 









