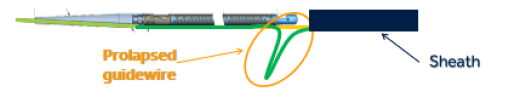
Medtronic Inc. is recalling its HawkOne Directional Atherectomy System product due to the risk of the guidewire within the catheter moving downward or prolapsing when force is applied during use.
January, 24, 2022 — Medtronic Inc. is recalling its HawkOne Directional Atherectomy System product due to the risk of the guidewire within the catheter moving downward or prolapsing when force is applied during use. If this happens, the catheter tip may break off or separate and this could lead to serious adverse events including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip.
The U.S. Food and Drug Administration (FDA) said there have been 163 complaints about this device issue. There have been 55 injuries and no deaths reported about this device issue.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
The HawkOne Directional Atherectomy System consists of a catheter and a cutter driver. This device is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
The recall includes 95,110 devices in the United States. These were distributed between January 2018 to Oct. 4, 2021. The vendor initiated the recall Dec. 6, 2021.
On Dec. 6, 2021, Medtronic sent an “Urgent Medical Device Notice" letter to customers requesting they:
• Share with all those who need to be aware within the organization or to any organization where the products have been transferred.
• Before using the HawkOne Directional Atherectomy System, review the “Instructions For Use” included with the product, noting the warnings and precautions listed in the “Urgent Medical Device Notice” letter.
• Complete the Customer Confirmation Form enclosed in the “Urgent Medical Device Notice" letter and email to [email protected].
Customers who have questions about this recall should contact their Medtronic Field Representative or call Medtronic Customer Service at (800) 854-3570.
Find lists of the effected product codes and lot numbers.
Find more atherectomy technology news
Find more cath lab technology news


 November 14, 2025
November 14, 2025 









