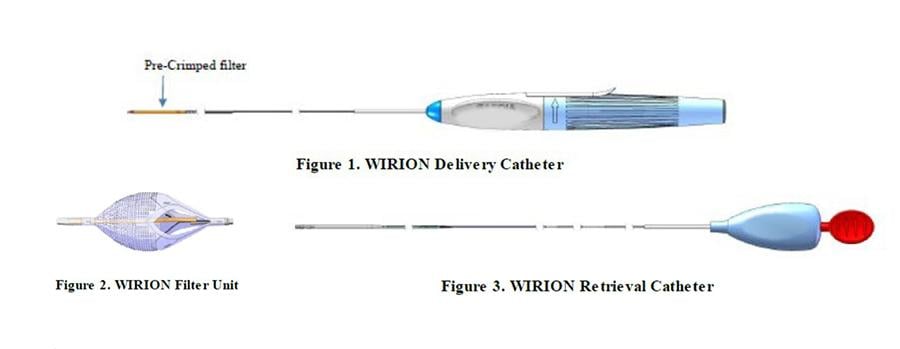
The CSI Wirion atherectomy embolic protection device is being recalled due to complaints of filter breakage during retrieval.
January 10, 2022 — Cardiovascular Systems Inc. (CSI) is recalling the Wirion atherectomy embolic protection device system due to complaints of filter breakage during retrieval. Under certain circumstances, such as when the filter basket is too full, the filter assembly may become difficult to withdraw. In this situation, withdrawal may cause the Wirion system filter component to tear or separate, which may result in series adverse events such as embolization or the need for additional medical procedures.
Cardiovascular Systems Inc. has received reports of nine device malfunctions, but no reports of death related to this device issue.
The U.S. Food and Drug Administration (FDA) has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
The Wirion system is used to hold debris or blood clots removed from the lower limbs during atherectomy.
There are 697 devices being recalled in the U.S. This includes all products and lots from Jan. 3, 2021 to Aug. 16, 2021. Distribution of the devices occurred between March 22, 2021 to Nov. 15, 2021.
The company initiated the recall Nov. 22, 2021, when CSI sent an urgent notification recall letter to customers instructing them remove the device from distribution and return the device to Cardiovascular Systems Inc.
Customers in the U.S. with questions about this recall should contact Cardiovascular Systems Inc. by phone at (65) 259-2800.
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.
For more information: www.csi360.com
Find more atherectomy systems news
FDA Issues Final Guidance on Peripheral Vascular Atherectomy Devices


 November 14, 2025
November 14, 2025 









