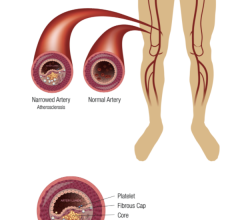
Sept. 1, 2024 — September is Peripheral Artery Disease (PAD) Awareness Month, a time to educate and empower people to ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
July 29, 2024 — CorVascular, a leading producer of peripheral arterial disease (PAD) / peripheral vascular disease (PVD) ...
July 11, 2024 — Medical device company R3 Vascular Inc., developer of novel, best-in-class bioresorbable scaffolds for ...
May 31, 2024 — Johnson & Johnson announced it has completed its acquisition of Shockwave Medical. Shockwave is now part ...
May 30, 2024 — Biotronik announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC ...
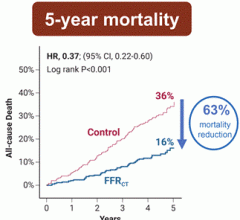
May 16, 2024 — A recent publication in the American Heart Association Circulation highlights a comprehensive ...
May 16, 2024 — Timely diagnosis and proper management of peripheral artery disease (PAD), including coordinated care ...
May 8, 2024 — In a groundbreaking development, a study published in the Journal of Vascular Surgery reveals for the ...
April 24, 2024 — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food ...
April 17, 2024 — Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast covered ...
April 11, 2024 — One-year success rates from angioplasty procedures to open clogged arteries in the legs were ...
April 5, 2024 — Johnson & Johnson and Shockwave Medical, Inc. today announced that they have entered into a definitive ...
March 27, 2024 — Elixir Medical has announced it has been granted Breakthrough Device Designation by the U.S. Food and ...
March 20, 2024 — Biotronik has been granted Breakthrough Device Designation (BDD) from the US Food and Drug ...
February 14, 2024 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, today announced that ...


 September 04, 2024
September 04, 2024