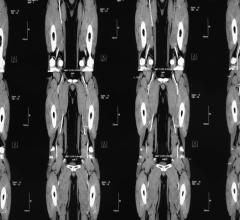
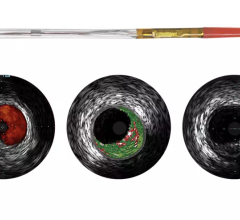
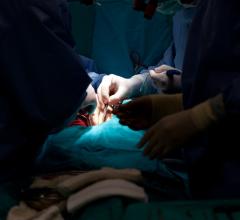
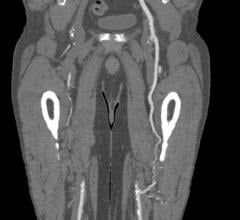
May 12, 2023 — The US FDA has granted an Investigational Device Exemption (IDE) approval for Concept Medical Inc’s novel ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
April 25, 2023 — FastWave Medical, a privately-held company founded by Big Sky Biomedical partners, announces the ...
March 8, 2023 — Treatments for peripheral artery disease (PAD) were largely developed in men and are less effective in ...
March 5, 2023 — Patients with cardiovascular disease, such as heart disease, stroke or peripheral arterial disease, were ...
February 16, 2023 — Northeast Scientific Inc., the pioneers in reprocessing single use peripheral vascular catheters ...
January 19, 2023 — Shockwave Medical, Inc., a pioneer in the development of Intravascular Lithotripsy (IVL) to treat ...
January 11, 2023 — In recent years, transcatheter mitral valve replacement (TMVR) treatment and technology has evolved ...
December 29, 2022 — Modern Vascular, the national leader in minimally invasive treatment for Peripheral Artery Disease ...
December 23, 2022 — According to Coherent Market Insights, the global Drug Eluting Stents market is estimated to be ...
November 15, 2022 Performing open bypass surgery to restore circulation for people with a severe form of peripheral ...
November 15, 2022 — Telehealth is a proven and valuable option for people with cardiovascular disease, however, there ...
October 11, 2022 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, announced that it ...
August 23, 2022 — The first US patient has been enrolled in the FDA SELUTION4BTK (Below-the-Knee) clinical trial ...
August 19, 2022 — Hackensack Meridian Health, Hackensack University Medical Center cardiac surgeon Yuriy Dudiy, M.D. be ...
July 28, 2022 — Northeast Scientific Inc., pioneers in reprocessing single use peripheral vascular catheters, announced ...

 May 12, 2023
May 12, 2023