November 6, 2012 — Vascular Solutions launched the R-Band radial hemostasis compression device in the United States. Vascular Solutions is the exclusive distributor of the R-Band device in the United States under an agreement entered into with Lepu Medical Technology (Beijing) Co. Ltd., developer and manufacturer of the device. Vascular Solutions introduced the R-Band to the clinical community at the 21st annual Transcatheter Cardiovascular Therapeutics (TCT) conference in Miami.
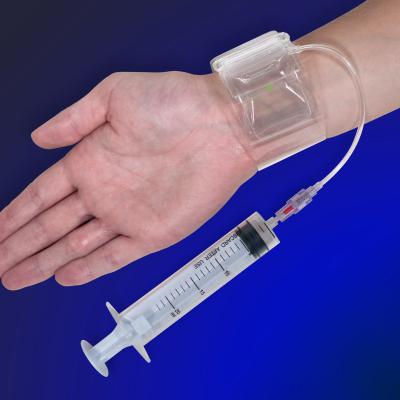
The R-Band, which has received U.S. Food and Drug Administration (FDA) 510(k) clearance, is a radial compression device that is used by interventional cardiologists to achieve hemostasis following transradial diagnostic and interventional catheterization procedures. The device consists of a soft plastic wrist strap with adjustable hook and loop fasteners, two inflatable compression balloons and an inflation syringe with a proprietary connector tip. After applying the strap around the patient's wrist, the physician inflates the compression balloons to apply gentle pressure to the puncture site of the radial artery, while maintaining flow through the ulnar artery. The clear plastic strap and clear compression balloons allow the clinician to visualize the access site during the entire process.
"R-Band will be an important addition to our product portfolio as we focus on serving the needs of the fast-growing radial artery access market and adding to our hemostat products business segment," said Howard Root, CEO of Vascular Solutions. "Radial access is undergoing tremendous expansion in the U.S., and we are especially pleased that the addition of the R-Band will allow us to offer a broader range of products for radial hemostasis to fit physician preferences, supplementing our existing Rad-Band and D-Stat Rad-Band products. In addition, the R-Band provides obvious synergies with the Accumed wrist positioning splint that we acquired in June."
Adoption of radial access has become a priority for many cardiac catheterization labs in the United States due to a growing acceptance of improved patient comfort, the availability of specialized radial devices and catheters, and the integration of radial access into practice guidelines and physician fellowship programs. According to a study by the Society for Cardiovascular Angiography and Interventions (SCAI), radial access accounted for under 3 percent of all diagnostic and interventional cardiac cath lab procedures in 2009. Based on recent market research conducted by Vascular Solutions, it is estimated that radial access now accounts for more than 12 percent of all such procedures in the United States. This level of penetration still significantly lags other parts of the world, such as Canada at 50 percent or the average for Europe of approximately 30 percent.
For more information: www.vasc.com


 October 27, 2021
October 27, 2021 







