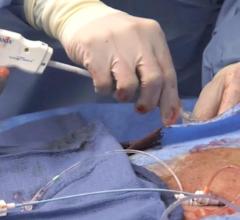
February 7, 2013 — Cardiva Medical Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Premarket Approval (PMA) for the Vascade Vascular Closure System (VCS). Clinical data from a prospective, multi-center, randomized 420 patient trial demonstrated that the Vascade VCS is clinically and statistically superior in both safety and efficacy compared to manual compression, the gold standard for vascular closure for patients undergoing percutaneous procedures through the femoral artery.
"We are thrilled to receive PMA approval for VASCADE," said Charles Maroney, president and CEO. Maroney continued, "We appreciate the effort of our clinical investigators who worked with us to achieve this important milestone and establish a new third generation closure technology. Cardiva is well positioned to bring this technology to the marketplace to increase patient throughput and provide a safer and more effective alternative to manual compression across the United States."
James Hermiller, M.D. of The Heart Center, Indianapolis, who served as principal investigator for the trial said, "I am extremely pleased with the outstanding clinical performance of Vascade and with the extraordinary contributions by over 65 clinical investigators and their staff at over 20 clinical sites in the U.S. and Australia who facilitated the study. With PMA approval, Vascade will now be available in the U.S. to all physicians who need safe and effective vascular closure."
Frank Zidar, M.D., clinical investigator at The Heart Hospital of Austin commented, "Vascade is a new generation of extravascular closure technology that offers significant advantages over conventional closure devices and manual compression. I am excited to be able to provide Vascade to my patients and to integrate it into my practice."
Vascade, an extravascular closure device, utilizes a unique delivery system based on a proprietary collapsible disc technology that provides temporary hemostasis during the procedure, which eliminates the need for an intra-vascular component. Secure and rapid hemostasis is achieved with Vascade by the deployment of a thrombogenic resorbable collagen patch at the arteriotomy of the femoral artery.
For more information: www.cardivamedical.com


 October 27, 2021
October 27, 2021