December 29, 2023 — Boston Scientific Corporation has initiated the AVANT GUARD clinical trial to evaluate the safety ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
December 26, 2023 — Pulse Biosciences, Inc., a company primarily focused on leveraging its novel and proprietary CellFX ...
December 22, 2023 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care through the power ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
December 22, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & ...
December 19, 2023 — Peerbridge Health has announce the successful completion and findings of its prospective ...
December 14, 2023 — Medtronic plc, a global leader in healthcare technology, today announced that the United States Food ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
December 14, 2023 — Individuals infected with COVID-19 are also at an increased risk of suffering from heart rhythm ...
December 13, 2023 — A new artificial intelligence (AI) model designed by Scripps Research scientists could help ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
December 8, 2023 — The American College of Cardiology (ACC) and the American Heart Association (AHA), along with several ...
December 7, 2023 — Ajax Health announced today the formation and funding of Cortex, a medical technology company ...
December 1, 2023 — As the use of wearable technology grows, smartwatches are marketed across the globe to consumers as a ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
November 30, 2023 — Johnson & Johnson MedTech1 today announced the completion of the acquisition2 of Laminar, Inc., a ...
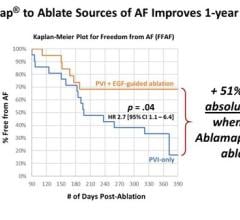
November 24, 2023 — During the American Heart Association Scientific Sessions 2023, held recently in Philadephia, PA, Mi ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...


 December 29, 2023
December 29, 2023