April 24, 2025 – The Heart Rhythm Society (HRS) has unveiled new research that underscores the critical role of ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
April 23, 2025 – The Heart Rhythm Society (HRS) has released a framework outlining criteria for establishing an Atrial ...
April 7, 2025 — CardioVia, a medical device company specializing in advanced cardiac care solutions, has received U.S ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
March 29, 2025 — Anthos Therapeutics, Inc., presented two new analyses from its AZALEA-TIMI 71 study at the American ...
Broward Health recently announced it is now offering the Farapulse Pulsed Field Ablation System to treat atrial ...
March 1, 202 — One in three people worldwide will develop a potentially life-threatening heart rhythm disorder in their ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
Feb. 14, 2025 — Volta Medical, a health technology company developing artificial intelligence (AI) solutions to assist ...
Feb. 12, 2025 — On Feb. 11, 2025, at HonorHealth Research Institute, Rahul Doshi, M.D., cardiac electrophysiologist ...
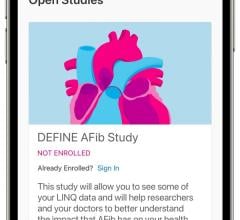
Jan. 16, 2025 — Primary results from the DEFINE AFib clinical study show the Medtronic LINQ family of insertable cardiac ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
Jan. 22, 2025 – Anthos Therapeutics, Inc., a clinical-stage biopharmaceutical company developing therapies to treat ...
Dec. 19, 2024 — Drinking multiple cups of coffee a day may help prevent cognitive decline in people with atrial ...
Nov. 18, 2024 — Abbott recently announced new data for the Amplatzer Amulet Left Atrial Appendage (LAA) Occluder to ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
Oct. 18, 2024 — The Heart Rhythm Society (HRS) Board of Trustees unanimously approved the formation of Heart Rhythm ...
Sept. 11, 2024 — In the first national estimate in two decades, researchers at the University of California-San ...
July 31, 2024 — A novel study co-authored by a heart failure cardiologist at University Hospitals Harrington Heart & ...


 April 25, 2025
April 25, 2025