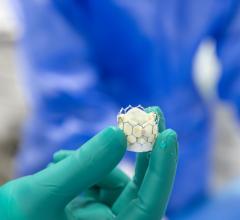
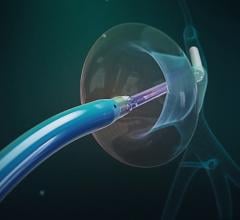
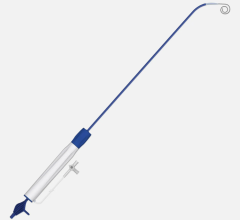
June 12, 2024 — Royal Philips, a global leader in health technology, announced the first implant of the Duo Venous Stent ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
June 12, 2024 — A team of Ochsner Health cardiologists recently published an article in the Journal of the American ...
June 11, 2024 — Stereotaxis, a pioneer in surgical robotics for minimally invasive endovascular intervention, today ...
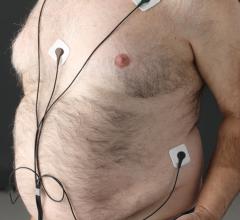
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

Innovation in medical technology exists to improve patient outcomes, increase surgical efficiency and enhance patient ...

June 7, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of the ...

Stroke recovery is a challenging process that extends for months after hospital discharge. Issues like cognitive ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

Here is a look at the Top 10 pieces of content viewers were reading during the month of May.
1. American College of ...
May 30, 2024 — Stereotaxis, a pioneer in surgical robotics for minimally invasive endovascular intervention, announced ...
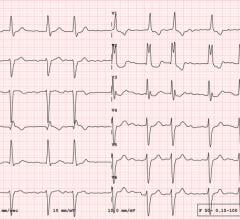
May 24, 2024 — An artificial intelligence (AI) program developed by investigators in the Smidt Heart Institute and their ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
May 21, 2024 — Stereotaxis, a pioneer and global leader in surgical robotics for minimally invasive endovascular ...
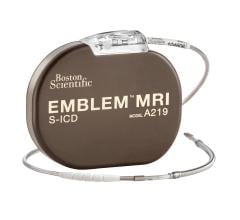
May 18, 2024 — Boston Scientific Corporation today announced positive six-month results from the ongoing pivotal MODULAR ...
May 18, 2024 — The Heart Rhythm Society (HRS) has announced the findings of three new studies demonstrating the safety ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 15, 2024 — A new study demonstrated parity between a minimally invasive procedure to replace the aortic valve in the ...
May 15, 2024 —CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac ...
May 13, 2024 — Innovative Health, Inc. announced that recent growth in single-use device reprocessing has delivered ...


 June 12, 2024
June 12, 2024