The American Heart Association recently announced that Dr. Christine E. Seidman, an internationally renowned physician ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
May 1, 2025 — Camgenium, a leading medical software engineering company, has announced further details of its ...
April 22, 2025 — Orchestra BioMed Holdings, a biomedical company accelerating high-impact technologies to patients ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
April 25, 2025 – Medtronic plc, a global leader in healthcare technology, recently received U.S. Food and Drug ...
April 27, 2025 – The Heart Rhythm Society (HRS) has announced the findings of new studies demonstrating the safety and ...
April 25, 2025 — The annual Society for Cardiovascular Angiography & Interventions (SCAI) meeting, SCAI 2025 Scientific ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
April 25, 2025 – Findings from two late-breaking clinical trials demonstrate the safety and efficacy of left bundle ...
April 24, 2025 – The Heart Rhythm Society (HRS) has unveiled new research that underscores the critical role of ...
April 23, 2025 – The Heart Rhythm Society (HRS) has released a framework outlining criteria for establishing an Atrial ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
April 24, 2025 – The Heart Rhythm Society (HRS) and the American College of Cardiology (ACC) have released a scientific ...
April 21, 2025 — Stereotaxis, a provider of surgical robotics for minimally invasive endovascular intervention, will ...
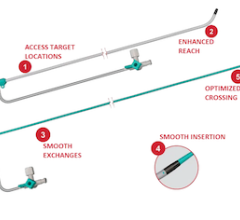
April 9, 2025 — Merit Medical Systems has announced the U.S. commercial release of its Ventrax Delivery System.
Ventr ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
April 7, 2025 — CardioVia, a medical device company specializing in advanced cardiac care solutions, has received U.S ...
March 29, 2025 — Anthos Therapeutics, Inc., presented two new analyses from its AZALEA-TIMI 71 study at the American ...
March 31, 2025 — iRhythm Technologies, Inc. has announced results from two large real-world retrospective analyses ...


 May 02, 2025
May 02, 2025