PLEASE NOTE: This webinar has been postponed to a later date.
A new date will be posted in the coming days.
...
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
...
February 16, 2024 — AMO Pharma Limited, a privately held clinical-stage specialty biopharmaceutical company focusing on ...
February 15, 2024 — Canary Medical, a medical data company focused on the development and commercialization of its ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
February 14, 2024 — The American College of Cardiology’s newest registry offers data-driven insights on cardiac ...
February 12, 2024 — BIOTRONIK, a leader in implantable medical device technology, announced today they will solely ...
February 12, 2023 — A team at Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital has successfully ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
February 7, 2024 — Combining brain stimulation with intense physical rehabilitation helped stroke survivors recover ...
February 6, 2024 — Cortex announced the initiation of its RESOLVE-AF trial (NCT05883631), a study formally launched in ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
February 2, 2024 — GE HealthCare (Nasdaq: GEHC) announces the latest innovation in electrophysiology (EP), the Prucka 3 ...
February 2, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson ...

It is a new year with lots of new content! Here is a look at the most-read content during the month of January on dicard ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
January 31, 2024 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration (FDA) appro ...
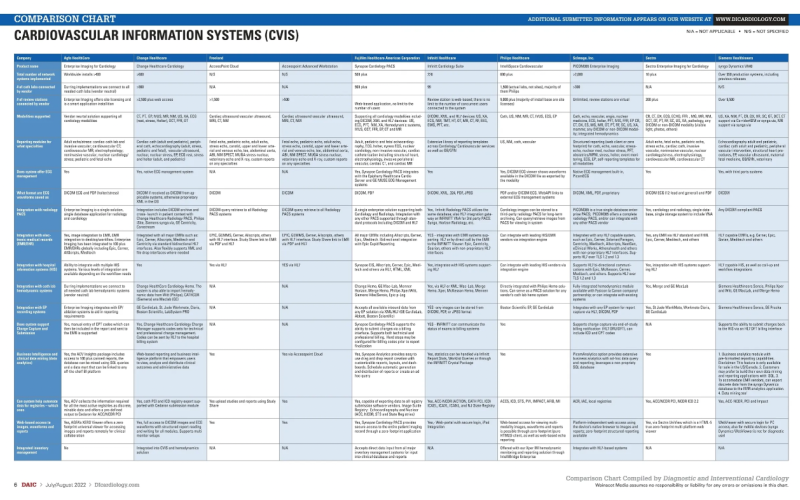
Did you know that Diagnostic and Interventional Cardiology (DAIC) maintains more than 40 comparison charts of product ...
January 19, 2024 — Orchestra BioMed, a biomedical company accelerating high-impact technologies to patients through risk ...