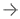May 15, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott is recalling the HeartMate 3 LVAS by updating the instructions for use after complaint reviews identified blood leakage ...
FDA
May 17, 2024 — Implicity, a leader in remote patient monitoring and cardiac data management solutions, announced it has ...
May 15, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott is recalling the HeartMate 3 LVAS by ...
May 8, 2024 — The US Food and Drug Administration (FDA) is alerting health care providers and facilities about our ...
May 8, 2024 — 4C Medical Technologies, Inc. ("4C Medical"), a medical device company dedicated to advancing minimally ...

Clinical trials and innovative technology took center stage during the month of April, racking up some record number ...
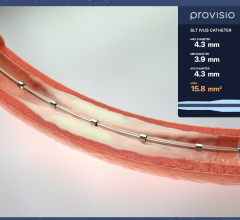
April 25, 2024 — Provisio Medical announced FDA 510(k) clearance of the Provisio SLT IVUS System. Sonic Lumen Tomography ...
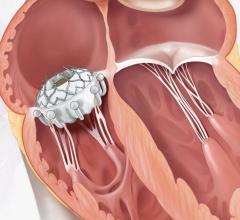
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using Edward ...
April 24, 2024 — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food ...
April 22, 2024 — At the annual American College of Cardiology conference (ACC.24) in Atlanta last week, RCE Technologies ...
April 18, 2024 — Bayer AG and Asklepios BioPharmaceutical, Inc., a gene therapy company wholly owned and independently ...
April 15, 2024 — The U.S. Food and Drug Administration (FDA) announced Abbott/Thoratec Corp. is recalling HeartMate II ...
April 12, 2024 — Simpson Interventions, Inc., a pioneering medical technology company specializing in cardiovascular ...
April 9, 2024 — UC Davis Health cardiology team members are among the first in the country to treat patients with tricus ...
April 4, 2024 — The U.S. Food and Drug Administration (FDA) announced that Medos International Sàrl is recalling Cerenov ...




 May 17, 2024
May 17, 2024