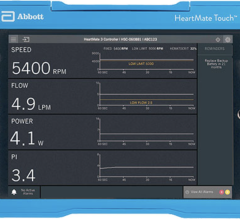
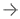March 13, 2024 — In the largest randomized clinical trial and first of its kind to date in the United States, a team led ...
FDA
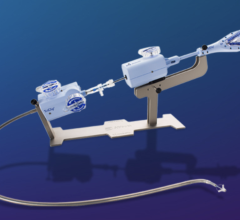
March 12, 2024 — Getinge has announced the U.S. Food and Drug Administration’s (FDA) 510(k) clearance of the Vasoview ...
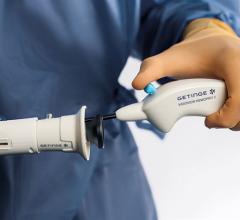
March 12, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott is recalling its HeartMate Touch ...
March 8, 2024 — Novo Nordisk today announced that the U.S. Food and Drug Administration (FDA) has approved an additional ...
March 7, 2024 — BioCardia, Inc. , a biotechnology company focused on advancing late-stage cell therapy interventions for ...
March 6, 2024 — Cleerly, the company on a mission to create a new standard of care to aid in the diagnosis of heart ...
March 6, 2024 — Ensight-AI, a Yale start-up and leader in AI-based cardiovascular diagnostics, announces the receipt of ...
March 6, 2024 — Lantheus Holdings, Inc. (Lantheus), a radiopharmaceutical company, has announced that the U.S. Food and ...
March 1, 2024 — Boston Scientific Corporation today announced it has received U.S. Food and Drug Administration (FDA) ...
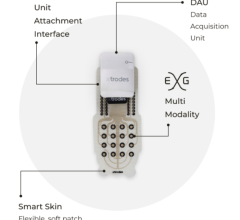
February 20, 2024 — X-trodes, a bio-convergence company bringing wireless monitoring solutions to the home environment ...
February 15, 2024 — Abbott announced that the Circulatory System Devices Panel of the Medical Devices Advisory Committee ...
February 13, 2024 — Butterfly Network, Inc., a digital health company transforming care through the power of portable ...
February 7, 2024 — Combining brain stimulation with intense physical rehabilitation helped stroke survivors recover ...
February 6, 2024 — BridgeBio Pharma, Inc., a commercial-stage biopharmaceutical company focused on genetic diseases and ...
February 2, 2024 — The American College of Cardiology’s Cardiac Oncology Conference, “Advancing the Cardiovascular Care ...


 March 13, 2024
March 13, 2024