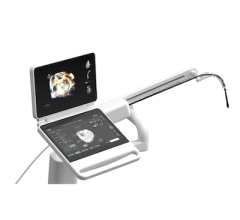
Sept. 04, 2025 — CorVista Health has published a new case series in the Journal of the American College of Cardiology ...
Sept. 9, 2025 — Neurescue ApS has received CE Mark approval for its Neurescue device, a medical device approved to treat ...
Sept. 8, 2025 — GE HealthCare recently announced a Distribution and Services Agreement (DSA) with CardioNavix, a part of ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Sept. 8, 2025 — Pulse Biosciences, Inc., a company leveraging its novel and proprietary Nanosecond Pulsed Field Ablation ...
Sept. 8, 2025 — AMO Pharma Ltd., a clinical-stage specialty biopharmaceutical company focusing on rare genetic disorders ...
Sept. 5, 2025 — Idorsia Ltd has announced a first-of-its-kind initiative with the Stanford Hypertension Center and Duke ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Sept. 4, 2025 — Royal Philips has introduced a new telemetry platform designed to help address critical challenges in ...
Sept. 2, 2025 —Corsera Health, Inc. has announced its launch with a mission to extend healthspan through cardiovascular ...
Sept. 2, 2025 — Johnson & Johnson MedTech has announced acute safety and effectiveness results from the Varipure ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Sept. 2, 2025 — Stereotaxis has announced the successful completion of the world’s first procedures using MAGiC Sweep, a ...
Sept. 3, 2025 — Laza Medical, Inc., a developer of AI-powered robotic ultrasound image guidance technology, has ...
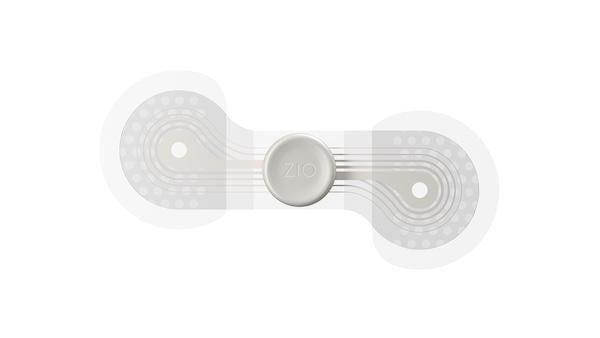
Patients with type 2 diabetes (T2D) and chronic kidney disease (CKD) are at elevated risk for cardiovascular disease.1 ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Sept. 3, 2025 — Kardium Inc. recently announced it has received pre-market approval (PMA) for the Globe Pulsed Field ...
Sept. 2, 2025 — Imperative Care, Inc. has announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its ...
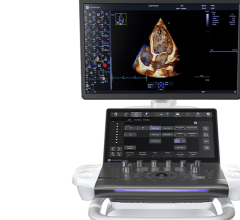
Aug. 29, 2025 — GE HealthCare has launched Vivid Pioneer, its most advanced, ultra-premium and adaptive cardiovascular ...


 September 09, 2025
September 09, 2025