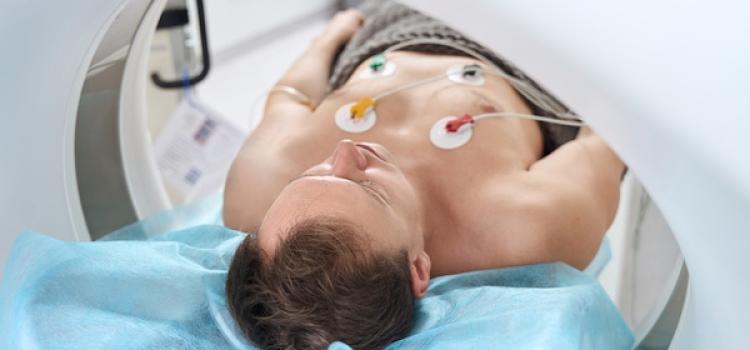
Advances in coronary CT angiography (CCTA) over the past two decades have helped cardiologists detect heart disease in asymptomatic patients more accurately. Now, providers are using dedicated cardiac ...
Feb. 12, 2026 — ACIST Medical Systems, Inc., a Bracco Group company, has announced the launch in selected markets in ...
Feb. 12, 2026 – CellProthera has acquired the transendocardial catheter originally developed by Celyad Oncology. This ...
Feb. 11, 2026 – Columbia HeartSource, an extension of Columbia University Irving Medical Center’s Department of Surgery ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Feb. 11, 2026 — The Northwestern Medicine Bluhm Cardiovascular Institute (BCVI) in Chicago has received a $50 million ...
Feb. 5, 2026 — New research shows that the use of an AI-enabled digital stethoscope more than doubled the identification ...
Feb. 4, 2026 — Acclaimed actor and philanthropist Jeremy Renner will serve as the closing speaker at the HIMSS Global ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Feb. 6, 2026 — Rapid Medical, a developer of active endovascular devices, has announced the latest results from the ...
Feb. 9, 2026 — Argá Medtech has announced positive clinical results from the BURST-AF (NCT05572047) first-in-human trial ...
Feb. 9, 2026 — GE HealthCare has announced the United States launch of ReadyFix, a remote fleet management solution ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Feb. 9, 2026 — Atraverse Medical presented new clinical and preclinical data at AF Symposium 2026 in Boston, further ...
Feb. 6, 2026 — Robert Wood Johnson University Hospital (RWJUH), in partnership with Rutgers Robert Wood Johnson Medical ...
Feb. 6, 2026 — Abbott has announced new clinical data from two late-breaking presentations at AF Symposium in Boston ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
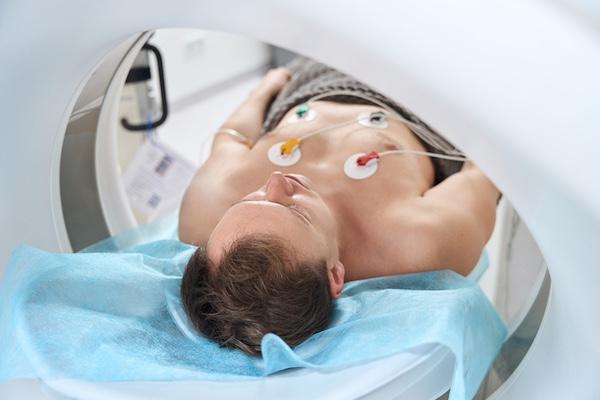
Advances in coronary CT angiography (CCTA) over the past two decades have helped cardiologists detect heart disease in ...
Feb. 3, 2026 — The first official summit of the European Association of Percutaneous Cardiovascular Interventions (EAPCI ...
Feb. 3, 2026 — Medtronic plc announced it will exercise its option to acquire CathWorks, a privately held medical device ...


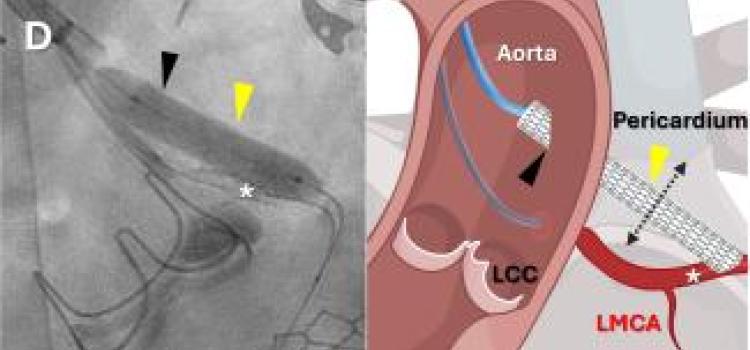
 February 12, 2026
February 12, 2026

















