Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave Javelin Peripheral IVL Catheter, a novel Forward IVL platform designed to modify ...
Catheters
Feb. 12, 2026 – CellProthera has acquired the transendocardial catheter originally developed by Celyad Oncology. This ...
Feb. 9, 2026 — Argá Medtech has announced positive clinical results from the BURST-AF (NCT05572047) first-in-human trial ...
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave ...
Oct. 23, 2025— Emboline, Inc., a provider of full-body embolic protection devices for transcatheter procedures, thas ...
Oct. 22, 2025 — Nyra Medical, a leading innovator in structural heart therapies, has announced the initiation of its ...
Aug. 28, 2025 — Medtronic plc has announced it received U.S. Food and Drug Administration (FDA) approval for the ...
July 1, 2025 — Stereotaxis, a provider of surgical robotics for minimally invasive endovascular intervention, has ...
May 21, 2025 – Johnson & Johnson MedTech has announced the U.S. launch of the Soundstar Crystal Ultrasound Catheter for ...
April 24, 2025 – The Heart Rhythm Society (HRS) and the American College of Cardiology (ACC) have released a scientific ...
July 17, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of ...
June 13, 2024 — The U.S. Food and Drug Administration (FDA) announced that Teleflex, and their subsidiary Arrow ...
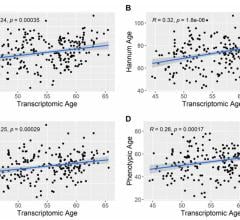
May 21, 2024 — A new research paper was published in Aging (listed by MEDLINE/PubMed as "Aging (Albany NY)" and "Aging ...





 February 12, 2026
February 12, 2026