Catheters
January 3, 2023 — The market has been studied for the below mentioned-segmentation and regional analysis for North ...
December 5, 2022 — The U.S. Food and Drug Administration (FDA) is alerting health care facilities and providers of a ...
September 21, 2022 — Shockwave Medical, Inc., a pioneer in the development of Intravascular Lithotripsy (IVL) to treat ...
August 25, 2022 — Imperative Care, Inc. announced that new data evaluating the utility of the Zoom Stroke Solution were ...
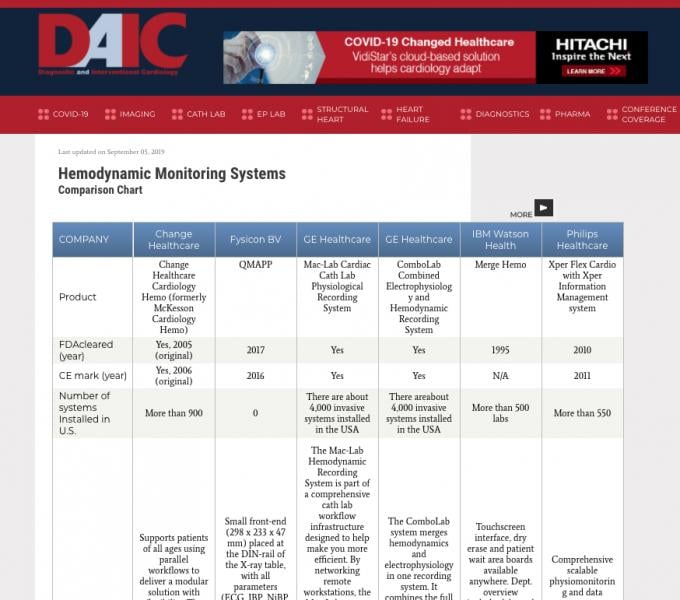
Diagnostic and Interventional Cardiology (DAIC) maintains more than 50 comparison charts of product specifications from ...
According to a new report from Allied Market Research, the global catheters market was valued at $22.7 billion in 2021 ...
May 26, 2022 — The U.S. Food and Drug Administration (FDA) has recalled the Dragonfly OpStar Imaging Catheter, and has ...
May 25, 2022 — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food ...
May 19, 2022 — Initial findings from the Distal versus Proximal Radial Artery Access for Cardiac Catheterization and ...
May 10, 2022 — The MIVI Neuroscience Q Aspiration Catheter incorporates a novel pusher wire design on its proximal end ...
April 21, 2022 – MIVI Neuroscience, Inc., innovator of the next generation of neurointerventional medical devices, today ...
April 12, 2022 – Siemens Healthineers, the global leader in intracardiac echocardiography (ICE), announced the market ...

In the electrophysiology (EP) lab, hundreds of thousands of used devices are sent to reprocessors every year to get ...


 January 31, 2023
January 31, 2023