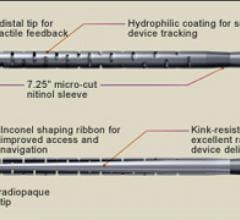
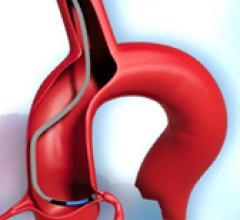
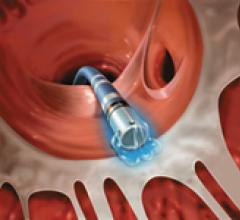
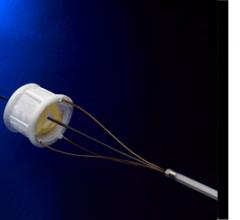
May 19, 2011 - BridgePoint Medical Inc. has announced that they have received clearance of an expanded indication for ...
Catheters
April 5, 2011 – A microcatheter system has received the CE mark. The Plato Microcath system, by Scientia Vascular ...
March 10, 2011 – A newly introduced catheter provides a superior answer for percutaneous fluid aspirations and ...
February 16, 2011 – To expand its portfolio of devices for lower extremity peripheral artery disease (PAD) ...
January 25, 2011 – A microcatheter offering superior crossability, flexibility and guidewire support during ...
January 13, 2011 – A catheter that removes emboli and thrombi from vessels in the coronary and peripheral ...
November 17, 2010 – A drug-eluting balloon (DEB) and a coronary stent have received CE mark approval in Europe ...
October 21, 2010 – Vascular Solutions has acquired the assets related to snare products from Radius Medical ...
October 13, 2010 – A new guidewire has been launched for use in challenging, small vessel peripheral angioplasty ...
October 5, 2010 – A U.S. clinical study evaluating the CrossBoss and Stingray catheters in treating chronically ...
September 28, 2010 – A guiding catheter specifically designed for right radial access to maximize transradial ...
September 27, 2010 - Treating varicose veins with a new access kit reduces the number of steps and reduces ...
September 21, 2010 – Two companies announced that they have entered into a distribution agreement in the United ...
July 16, 2010 – Biosense Webster Inc. recently announced that more than 10 patients have now been enrolled in the ...
June 24, 2010 – The National Institutes of Health (NIH) announced this week that it has given a $2.2 million grant ...

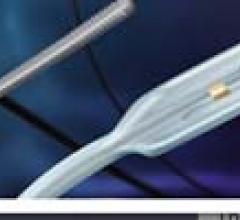
 May 19, 2011
May 19, 2011