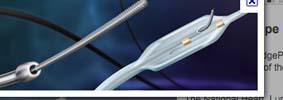
May 19, 2011 - BridgePoint Medical Inc. has announced that they have received clearance of an expanded indication for the CrossBoss Catheter and Stingray System from the U.S. Food and Drug Administration (FDA). The expanded indication includes the intraluminal placement of conventional guidewires beyond stenotic coronary lesions [including chronic total occlusions (CTOs)] prior to PTCA or stent intervention. CTOs are chronically stenosed lesions that completely block a coronary artery – typically for 3 months or longer – and prevent blood circulation to critical areas of the heart. This expanded indication is the result of data generated from the 147-patient FAST-CTOs clinical trial in which the safety and effectiveness of the system in coronary chronic total occlusions was demonstrated. William Lombardi, M.D., of St. Joseph's Hospital in Bellingham, Wash., and a leading enroller in the clinical trial states, "The BridgePoint system is the first set of devices designed specifically for treating coronary CTOs that has shown real improvements in safety, procedural efficiency, and clinical success rates. The clinical trial has proven that these arteries can be opened effectively with these products and now with the expanded indication from the FDA there will be a new level of awareness among physicians and patients." BridgePoint's ability to commercialize its CrossBoss Catheter and Stingray System will be greatly expanded with this new level of indication. BridgePoint is the first interventional device company to be granted this specific type of approval for both crossing and re-entry technologies to be used in the treatment of coronary CTOs. For more information: www.bridgepointmedical.com


 October 28, 2025
October 28, 2025 









