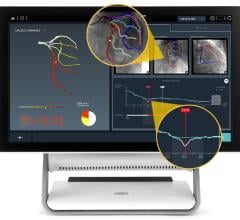
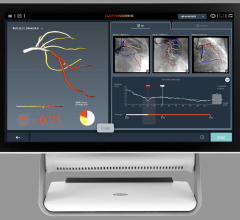
The global interventional cardiology device market is driven by the number of procedures performed across the globe. The market is primarily driven by the number of angiography and angioplasty ...
FFR Technologies
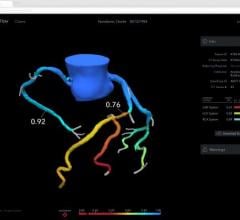
This channel includes news and new technology innovations for fractional flow reserve (FFR) wires, catheters and systems used to measure blood flow across a coronary lesion to determine if a stent is needed or if the plaque stenosis can be treated medically. The section includes iFR, instantaneous wave-free ratio, systems used in the cath lab and noninvasive FFR technologies including computed tomography-FFR. This is also referred to as CT-FFR or FFR-CT.
Feb. 3, 2026 — Medtronic plc announced it will exercise its option to acquire CathWorks, a privately held medical device ...
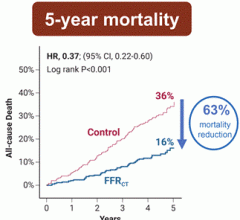
Oct. 27, 2024 — HeartFlow, Inc. recently announced seven-year data confirming the use of HeartFlow’s AI-enabled ...
July 17, 2024 — HeartFlow, a leader in non-invasive artificial intelligence (AI) heart care solutions, today announced ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 4, 2024 — HeartFlow, a leader in cardiovascular healthcare technology, is pleased to announce a key Medicare policy ...
May 23, 2024 — HeartFlow, Inc., a leader in non-invasive artificial intelligence (AI) heart care solutions, announced ...
May 8, 2024 — In a groundbreaking development, a study published in the Journal of Vascular Surgery reveals for the ...
January 23, 2024 — HeartFlow, Inc., a leader in non-invasive artificial intelligence (AI) precision coronary solutions ...
January 3, 2024 — HeartFlow, Inc., a leader in non-invasive artificial intelligence (AI) precision coronary care ...
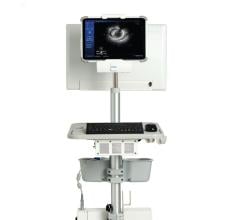
September 27, 2023 — Boston Scientific announced the FDA clearance of the AVVIGOTM+ Multi-Modality Guidance System, a ...
The global interventional cardiology device market is driven by the number of procedures performed across the globe. The ...
July 28, 2023 — Respective results from the REVEALPLAQUE, DECODE, and SMART-CT clinical studies demonstrate the accuracy ...
May 5, 2023 — According to an accepted manuscript published in ARRS’ own American Journal of Roentgenology (AJR), a high ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 3, 2023 —CathWorks announced key events for the company during the annual American College of Cardiology (ACC) ...




 February 03, 2026
February 03, 2026