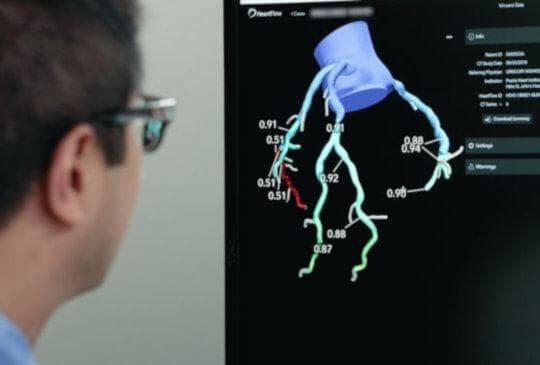
July 22, 2022 — HeartFlow, Inc., the leader in revolutionizing precision heart care, released two datasets utilizing its HeartFlow AI-based Plaque technology* (referred in below as “HeartFlow Plaque”). The first dataset - HeartFlow’s largest study to date - studied over 11,800 patients and enables physicians to understand a patient's burden of coronary plaque compared to their age and sex-matched peers. The second dataset demonstrated that HeartFlow Plaque* may be “a reasonable non-invasive alternative to invasive angiography for assessment of coronary plaque”.1 Both studies were presented at the 17th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography (SCCT) in Las Vegas, NV, July 15-17th, 2022.
The “Nomographic CT Quantitative Plaque Data from a Large International Population”, presented by Georgios Tzimas, MD, University of British Columbia, Providence Health Care supported the clinical utility of being able to distinguish patients with high or low volumes of plaque across a population. HeartFlow Plaque* was applied to over 11,800 coronary computed tomography angiograms (CCTAs) and atherosclerotic plaque burden data were stratified by age and sex. Understanding how an individual patient’s plaque volume compares to that of the general population can provide context for physicians as they consider the best treatment plan for an individual patient. The information may also help motivate patients to adhere to recommended medications or lifestyle modifications.
The “Quantitative Assessment Of AI-based CCTA Plaque Volume Compared With IVUS2” presentation by Kersten Petersen, PhD, Senior Manager, Research, showed that HeartFlow Plaque* agreed well with intravascular ultrasound (IVUS) measures of plaque volume (correlation coefficient of 0.92). This confirms that HeartFlow Plaque* from CCTA is accurate when compared to IVUS and shows a strong correlation across a wide range of plaque volumes and types. By accurately quantifying the amount of plaque present in a patient’s coronary arteries, physicians can be provided with meaningful quantitative plaque information from CT images.
“We’ve known for years that atherosclerosis and coronary risk are multifactorial, reflecting aspects both of plaque burden and composition, as well as physiological influences. Understanding both plaque burden and physiology are imperative to assessing patient risk and optimizing treatment plans for patients with coronary artery disease,” said Campbell Rogers, MD, FACC, Chief Medical Officer, HeartFlow. “The new data reflect the company’s belief in the value of precise plaque information being additive to the critical physiological data we provide through FFRCT. We look forward to introducing HeartFlow Plaque* and working with physicians to understand better the interplay of plaque and physiology across the spectrum of coronary disease.”
*Currently pending 510(k) clearance from the Food and Drug Administration (FDA). Not available for sale.
About the HeartFlow FFRct Analysis
Starting with a standard coronary computed tomography angiogram (CCTA), the HeartFlow Analysis leverages algorithms trained using deep learning (a form of AI) and highly trained analysts to create a digital, personalized 3D model of the heart. The HeartFlow Analysis then uses powerful computer algorithms to solve millions of complex equations to simulate blood flow and provides FFRct values along the coronary arteries. This information is used by physicians in evaluating the impact a blockage may be having on blood flow and determine the optimal course of treatment for each patient. A positive FFRct value (≤0.80) indicates that a coronary blockage is impeding blood flow to the heart muscle to a degree which may warrant invasive management.
Data demonstrating the safety, efficacy and cost-effectiveness of the HeartFlow Analysis have been published in more than 500 peer-reviewed publications, including long-term data out to five years.1 The HeartFlow Analysis offers the highest diagnostic performance available from a non-invasive test.3 To date, clinicians around the world have used the HeartFlow Analysis for more than 130,000 patients to aid in the diagnosis of heart disease.1
About HeartFlow Plaque* Overview
The HeartFlow Plaque* overview will provide plaque volume and characterize the type of plaque present. The HeartFlow Plaque* feature is based on a fully automated deep-learning (a form of AI) algorithm for characterizing and quantifying plaque. In an internal study, the HeartFlow Plaque* technology was found to be more reliable than expert CT readers in identifying different types of plaque and quantifying total plaque volume.4 By adding the plaque overview to the physiological information currently provided by the HeartFlow Analysis, physicians will gain a more comprehensive understanding of a patient’s coronary disease burden and support efficient risk stratification of patients who may be at high risk of death from a heart attack.
For more information: www.heartflow.com


 November 14, 2025
November 14, 2025 









