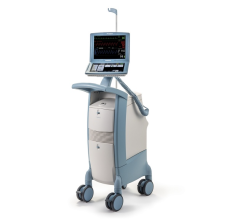
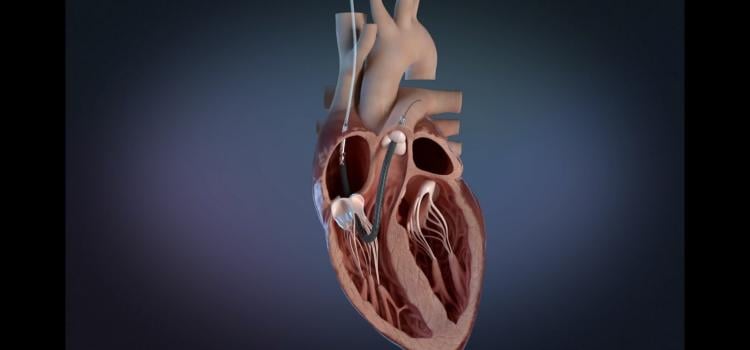
Jan. 8, 2026 — Compremium AG recently announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to its novel solution designed to directly measure central ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
Jan. 8, 2026 — Compremium AG recently announced that the U.S. Food and Drug Administration (FDA) has granted ...
July 10, 2024 — Reviva Pharmaceuticals Holdings, Inc., a late-stage pharmaceutical company developing therapies that ...
June 19, 2024 — When electrophysiologist Eugenio Cingolani, MD, isn’t seeing patients, he can usually be found in his ...
June 13, 2024 — Xeltis, a leading developer of transformative implants that enable the natural creation of living and ...
May 8, 2024 — The US Food and Drug Administration (FDA) is alerting health care providers and facilities about our ...
February 21, 2024 — Ochsner Children's Hospital, ranked among the top hospitals in the nation for pediatric cardiology a ...
December 5, 2023 — Raymond Benza, MD, FACC, FAHA, FACP, a top expert in cardiovascular medicine and advanced heart ...
October 31, 2023 — Tenaya Therapeutics, Inc., a clinical-stage biotechnology company with a mission to discover, develop ...
October 23, 2023 — Supira Medical, Inc., a Shifamed portfolio company, announced that the company's percutaneous ...
October 5, 2023 — A newly-issued "Scientific Statement on Durable Mechanical Circulatory Support" in the October issue ...
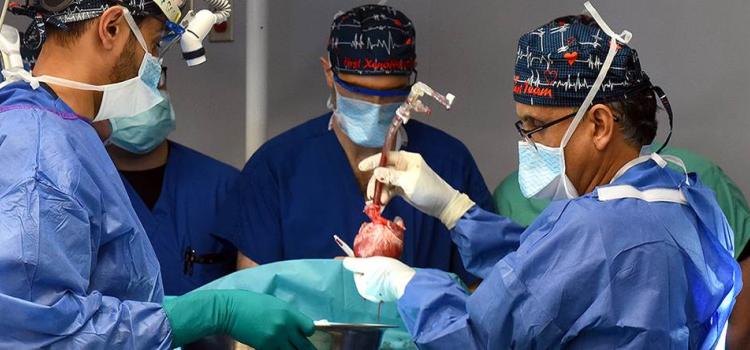
September 25, 2023 — After world’s first successful transplant in 2022, also performed at the University of Maryland ...
September 15, 2023 — A team of interventional cardiologists from Henry Ford Health’s Center for Structural Heart Disease ...
September 13, 2023 — The Smidt Heart Institute at Cedars-Sinai has earned a prestigious designation for its excellence ...
August 18, 2023 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the labeling for ...
August 14, 2023 — The FDA has announced that Datascope/Maquet/Getinge is recalling the Cardiosave Hybrid and Rescue ...

 January 13, 2026
January 13, 2026