Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic balloon pumps (IABP) will increasingly face a more serious challenge from small percutaneous left ventricular assist devices (pLVAD). This was reinforced when I received a cardiology market analysis from GlobalData agreeing with this assessment.
Since the 1970s, IABPs have been the gold standard for hemodynamic support in low ejection fraction patients, especially those with myocardial infarction complicated by cardiogenic shock. However, when IABPs were first introduced, there was no U.S. Food and Drug Administration (FDA) clinical trial requirement to prove efficacy. So, until now, only a few registry studies and clinical trials have shown IABPs can improve blood pressure and coronary perfusion. Based on this limited data, international guidelines recommend the use of IABPs for cardiogenic shock.
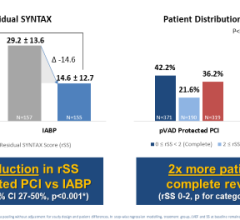
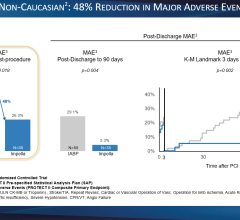
Use of IABPs for cardiogenic shock is estimated at 25-40 percent of cases. This low rate may be due to cardiologists who are still not entirely convinced of IABP efficacy. The IABP-SHOCK II trial aimed to provide evidence IABPs can reduce the 30-day mortality rate compared to standard medical therapy. But, instead of showing the superiority of IABP therapy, 30-day results from the large, randomized, multicenter trial did not meet the pre-specified 12 percent improvement in survival endpoint. Although IABPs proved to be safe, the study provided little evidence that they are associated with hemodynamic improvement, failing to reduce rates of mortality, and showing no improvement in blood pressure, no reduction in time in the intensive care unit and no improvement in organ perfusion.
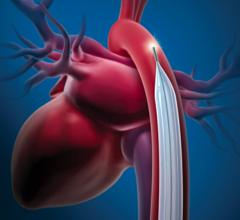
This data came out at the same time Abiomed announced FDA clearance for its improved Impella CP (Cardiac Power) pLVAD. It is the same size as the Impella 2.5 liter-per-minute device, but delivers up to 4 liters per minute, representing about 80 percent of the blood flow in a normal heart. The lower-output 2.5 device showed it provided more hemodynamic support than IABPs in previous Abiomed-sponsored studies (Maquet and Teleflex say their balloons provide between 0.5 and 1 liter of augmentation support). The higher-output Impella CP will place the device head and shoulders above the support IABPs can provide.
Despite the apparent advantages of pLVAD therapy (CardiacAssist’s TandemHeart and the Abiomed Impella), GlobalData said several randomized, controlled trials demonstrated a better hemodynamic profile compared to IABP, but pLVAD use did not translate into improved 30-day survival. The Impella CP may change this. But even if it does not, supporters of IABPs say their use increases perfusion, and in that regard the Impella CP has a verifiably higher ability to do so. Impella’s higher cost compared to IABPs will likely be the biggest barrier to wider adoption.


 August 14, 2023
August 14, 2023