Oct. 30, 2025 — Recor Medical and its parent company, Otsuka Medical Devices presented the results from two clinical studies presented at the recent 2025 Transcatheter Cardiovascular Therapeutics (TCT ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
Oct. 30, 2025 — Recor Medical and its parent company, Otsuka Medical Devices presented the results from two clinical ...
Oct. 23, 2025 — At TCT 2025, the Cardiovascular Research Foundation (CRF) announced the 2025 SET-10 (Scientific ...
Oct. 29, 2025 — FastWave Medical presented new first-in-human (FIH) and pre-clinical data for its Sola coronary laser ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
Oct. 27, 2025 — At the annual Transcatheter Cardiovascular Therapeutics (TCT 2025) meeting in San Francisco, Royal ...
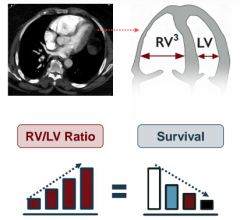
Oct. 27, 2025 — Results from the PREVUE-VALVE study suggest that there are currently at least 4.7 million people aged 65 ...
Oct. 25, 2025 — Medtronic plc has announced the launch of the Stedi Extra Support guidewire, designed to enhance ...
Oct. 27, 2025 — Elixir Medical, a developer of technologies to treat cardiovascular disease, has announced new clinical ...
Oct. 23, 2025— Emboline, Inc., a provider of full-body embolic protection devices for transcatheter procedures, thas ...
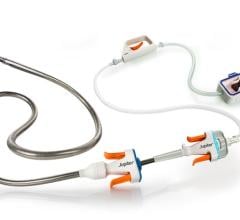
Oct. 26, 2025 – Jupiter Endovascular, Inc., a medical technology company developing a new class of endovascular ...
Oct. 27, 2025 – Penumbra, Inc. has announced the results of the STORM-PE randomized controlled trial (RCT), which found ...
Oct. 22, 2025 — Heartflow, Inc. has introduced Heartflow PCI Navigator, the newest addition to the Heartflow One ...
Oct. 22, 2025 — Nyra Medical, a leading innovator in structural heart therapies, has announced the initiation of its ...
Aug. 27, 2025 – The Cardiovascular Research Foundation (CRF) has announced the late-breaking clinical trials and science ...
June 12, 2025 – The American Society of Echocardiography (ASE) and Cardiovascular Research Foundation (CRF) have ...
May 5, 2025 — The TCT Geoffrey O. Hartzler Master Operator Award will be presented to Kenneth Rosenfield, MD, MSc ...






 November 03, 2025
November 03, 2025