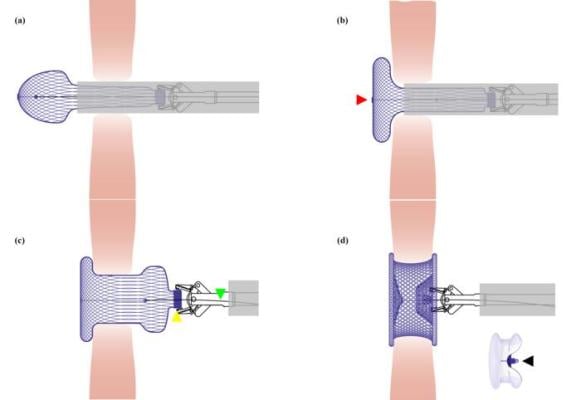
Illustration for the deployment and shaping process of the occluder. (a) The left side of the bioabsorbable occluder formed a spherical shape after being released from the delivery sheath. (b) The left side of the bioabsorbable occluder was plasticized from a spherical shape into a disc shape by pulling back the shape line, which was tied on the center of the left disc (indicated by the red triangle). (c) The right side was spherical after being released from the delivery sheath. The yellow triangle indicated the connecting rivet of the occluder. The green triangle indicated the connecting line. (d) The entire occluder was shaped into the ‘‘double-umbrella” framework by pulling back the shape line and simultaneously pushing the delivery cable. There was a knot (indicated by the black triangle) on the shape line, which would be pulled beyond the connecting rivet of the occluder to ensure the stability of the double-umbrella shape once released. ©Science China Press
July 14, 2023 — In recent years, transcatheter intervention techniques have emerged as a promising alternative for the closure of perimembranous ventricular septal defect (VSD). The advancements in trancatheter VSD closure have significantly contributed to its widespread adoption. Traditionally, VSD occluders are often made of shape memory alloys, such as nitinol. Although nitinol-based occluders are predominantly used in current clinical practice, they have drawbacks such as nickel release, and tissue erosion and compression, which may underlie severe complications such as allergy, cardiac perforation, and complete atrioventricular block. An occluder constructed from bioabsorbable materials is anticipated to overcome these limitations by utilizing a temporary scaffold that can be naturally absorbed by the body. The premise behind this approach is that the bioabsorbable scaffold provides an environment conducive to endothelization, enabling the formation of a native tissue barrier. However, current bioabsorbable occluders have necessitated the use of a metal frame to provide structural support, retain the occluder's shape, and ensure visibility during fluoroscopy, results in incomplete degradation of the occluder. To promote synchronized fully degradation and endothelialization, researchers developed a hybrid structure comprising a polydioxanone (PDO) monofilament double-umbrella framework with relatively rapid degradation and a poly-L-lactic acid (PLLA) fabric barrier-film with relatively slow degradation.
This study was a multicenter, randomized clinical trial to investigate the efficacy and safety of a fully biodegradable occluder in patients with perimembranous VSD. From April 2019 to January 2020, 125 patients in seven centers were screened and a total of 108 eligible participants were randomized into the bioabsorbable occluder group (n = 54 patients) and nitinol occluder group (n = 54). A non-inferiority designwas utilized and all patients underwent transcatheter device occlusion. The primary efficacy endpoint was the successful implantation rate of the occluder, along with the absence of residual shunt measuring less than 2 mm as assessed by transthoracic echocardiography (TTE) at the 6-month postoperative follow-up. The safety endpoint encompassed device-related complications within 24 months, including a composite of death, occluder displacement, new-onset moderate or severe valve regurgitation or worsening of pre-existing regurgitation, cardiovascular surgery due to adverse events, symptoms of embolization, or occurrence of arrhythmias.
All patients enrolled in the trial successfully underwent implantation and completed the study. The fully bioabsorbable occluder showed non-inferiority for the efficacy of VSD closure and safety endpoint. Throughout the follow-up period, no residual shunt greater than 2 mm was observed. Transthoracic echocardiography revealed a hyperechoic area corresponding to the bioabsorbable occluder, which primarily decreased within the first year after implantation and completely disappeared within 24 months. The only occluder-related complication observed was postprocedural arrhythmia, with an incidence of 5.56% in the bioabsorbable group and 14.81% in the nitinol group (P = 0.112). Notably, the incidence of sustained conduction block was lower in the bioabsorbable occluder group (0/54) compared to the nitinol group (6/54) at the 24-month follow-up (P = 0.036).
In conclusion, this novel fully bioabsorbable occluder with a hybrid structure utilizing PDO and PLLA demonstrates the non-inferior successful and safe implantation in patients with VSD. The incorporation of a hybrid structure, the utilization of the shape line, echocardiographic guidance, and the awareness of synchronous biodegradation collectively pave the way for the development of next-generation fully bioabsorbable occluders for other structural heart diseases.
For more information: https://www.sciengine.com/publisher/sc


 June 19, 2024
June 19, 2024 









