The cardiology program at Tufts Medical Center in Boston is internationally recognized for being on the forefront of ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
August 21, 2020 — The U.S. Centers for Medicare and Medicaid Services (CMS) proposed updates to coverage policies for to ...
August 5, 2020 — The U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) this week for ...
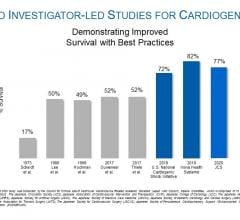
August 3, 2020 — A three-year, investigator-led, prospective study of Japanese patients who received an Impella heart ...
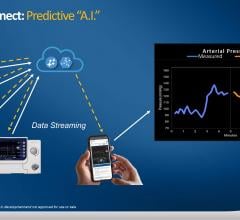
July 16, 2020 – The U.S. Food and Drug Administration (FDA) approved one-way digital data streaming during patient ...
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
July 6, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) magaz ...
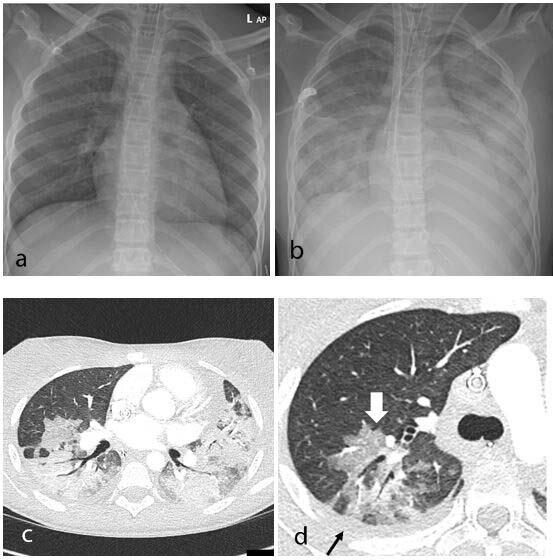
In recent weeks, a multisystem hyperinflammatory condition has emerged in children in association with prior exposure or ...
It was originally thought novel coronavirus (COVID-19, SARS-CoV-2) was primarily a respiratory disorder, but as larger ...
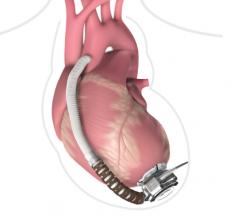
June 5, 2020 — Abiomed announced the U.S. Food and Drug Administration (FDA) has approved the company's investigational ...
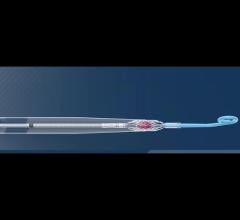
This is a quick animation demonstrating how the new 9 French Abiomed Impella ECP expands to approximately 18 French and ...
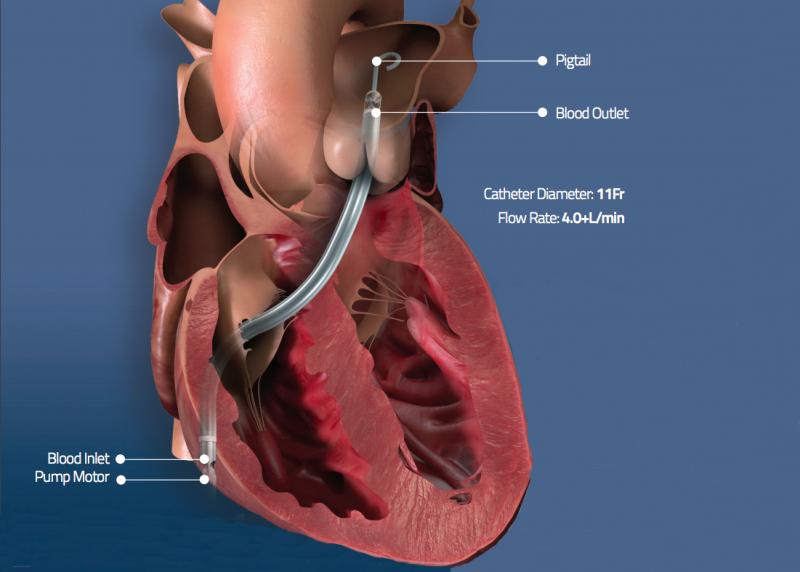
June 1, 2020 — The U.S. Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for the Abiom ...
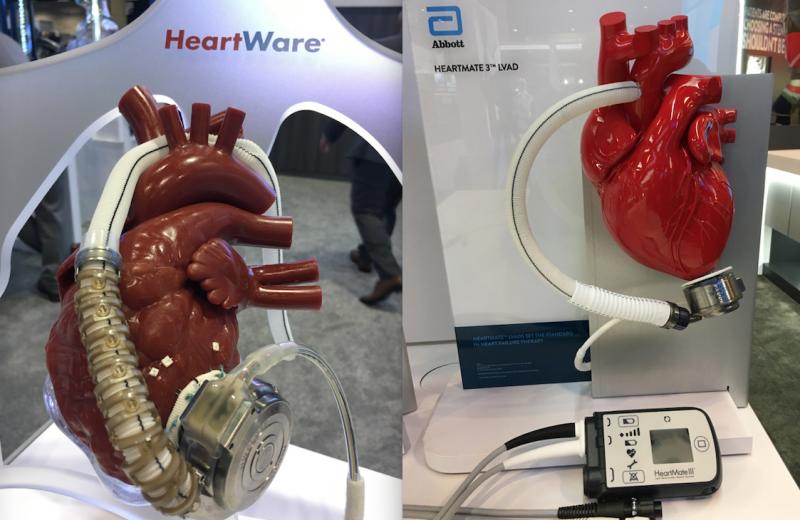
May 29, 2020 — Medtronic is recalling its HeartWare HVAD left ventricular assist device (LVAD) pump outflow graft and ...
May 27, 2020 — Carmat, a developer of the of a next generation advanced total artificial heart, announces the first ...
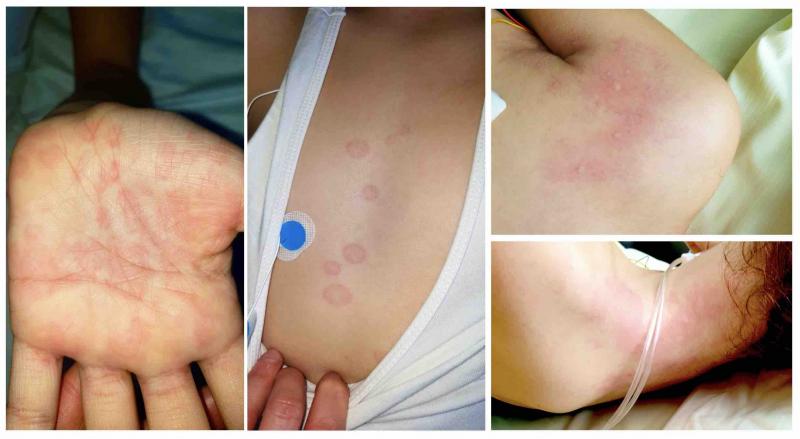
A new, serious COVID-19 cardiovascular presentation emerged in late April and early May 2020 in the form of pediatric ...


 August 21, 2020
August 21, 2020