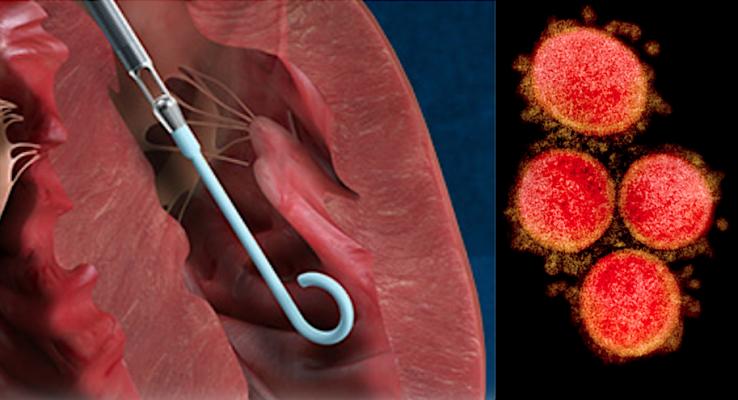
Abiomed Impella is now authorized to treat critical ICU COVID-19 patients on ECMO who develop pulmonary edema or need cardiac decompensation from myocarditis.
August 5, 2020 — The U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) this week for the use of Abiomed Impella left ventricular (LV) support systems to providing temporary LV unloading and support to treat critical coronavirus (COVID‐19) patients who are already on extracorporeal membrane oxygenation (ECMO) treatment.
The EUA specifies Impella pumps can be used if the COVID patient develops pulmonary edema while on veno-arterial (VA) ECMO support, or late cardiac decompensation from myocarditis while on veno-venous (VV) ECMO support.
This FDA authorization includes the use of the Impella 2.5, Impella CP, Impella CP with SmartAssist, Impella 5.0, and Impella 5.5 with SmartAssist percutaneous circulatory support systems for the left ventricle.
RADM Denise M. Hinton, chief scientist, FDA, wrote in the authorization letter that COVID-19 patients may require V-A ECMO for circulatory support with oxygenation due to acute cardiopulmonary failure. However, in some patients, V-A ECMO alone does not provide sufficient LV unloading due to retrograde aortic perfusion, which can lead to LV overload and distension, resulting in pulmonary edema. The FDA said this emergency use of the Impella devices can alleviate this problem by unloading the LV, thereby reducing the LV work, and fully emptying the LV in critical care patients.
In addition, COVID-19 patients may require V-V ECMO for pulmonary failure. Some of these patients suffer from LV decompensation from myocarditis, which may require additional mechanical circulatory support for systemic perfusion. For these patients, the Impella LV Support Systems may be effective at providing the necessary LV support for hemodynamic stability and end-organ perfusion.
Hinton said conditions of pulmonary edema and late cardiac decompensation from myocarditis during ECMO therapy requiring temporary LV support are substantially different than the conditions of high-risk percutaneous coronary intervention (PCI) and cardiogenic shock requiring temporary LV support, for which the Impella systems are FDA-approved to treat. However, based on the available information, including extrapolated data from the approved indications and reported clinical experience, FDA has concluded that the Impella systems may be effective at providing temporary LV support for the treatment of pulmonary edema and late cardiac decompensation in critical care ICU COVID patients.
Criteria for Issuance of Authorization for Impella in COVID Patients on ECMO
Hinton said this emergency use of the Impella pumps meets the criteria set forth by the U.S. Department of Health and Human Services (HHS) March 2020 Declaration that Circumstances Exist Justifying Authorizations due to COVID-19. These include:
1. SARS-CoV-2, the disease that causes COVID-19, can cause a serious or life-threatening disease or condition, including severe respiratory illness, to humans infected by this virus;
2. Based on the totality of scientific evidence available to FDA, it is reasonable to believe that the Impella LV support systems may be effective when used by healthcare professionals in the hospital setting for providing temporary LV unloading and support to treat critical care patients with confirmed COVID-19 infection who are undergoing ECMO treatment and who develop pulmonary edema while on V-A ECMO support or late cardiac decompensation from myocarditis while on V-V ECMO support, and that the known and potential benefits of the Impella systems for such use outweigh the known and
potential risks; and,
3. There is no adequate, approved, and available alternative to the emergency use of the Impella LV support systems for critical care patients undergoing ECMO treatment for
COVID‐19 who develop pulmonary edema while on V-A ECMO support or late cardiac
decompensation from myocarditis while on V-V ECMO support.
FDA Scope of Authorized Uses for the Impella in Patients on ECMO
The scope of this authorization limits the use of the Impella pumps to four days days for Impella 2.5, Impella CP, and Impella CP with SmartAssist. It limits the use to 14 days for Impella 5.0 and Impella 5.5 with SmartAssist.
The FDA stated Impella pumps not intended for use to treat patients with the following conditions:
• Mural thrombus in the left ventricle;
• Presence of a mechanical aortic valve or heart constrictive device;
• Aortic valve stenosis/calcification (equivalent to an orifice area of 0.6 cm2 or less);
• Moderate to severe aortic insufficiency (echocardiographic assessment graded as ≥ +2);
• Severe peripheral arterial disease precluding placement of the Impella System;
• Significant right heart failure;
• Combined cardiorespiratory failure;
• Presence of an Atrial or Ventricular Sepal Defect (including post-infarct VSD);
• Left ventricular rupture; or
• Cardiac tamponade.
Read the full FDA emergency use authorization letter.
Read more about FDA emergency use authorizations for COVID-19
Related COVID-19 Hemodynamic Support Content:
Impella RP Granted FDA Emergency Use Authorization for COVID-19 Patients With Right Heart Failure
FDA Approves ECMO to Treat COVID-19 Patients
LivaNova Modifies its ECMO Indications Beyond Six Hours to Address COVID-19
The Cardiovascular Impact of COVID-19
VIDEO: Multiple Cardiovascular Presentations of COVID-19 in New York — Interview with Justin Fried, M.D., explaining a case that used VV-ECMO abnd VAV-ECMO
VIDEO: Impact of COVID-19 on the Interventional Cardiology Program at Henry Ford Hospital — Interview with William O'Neill, M.D.
Kawasaki-like Inflammatory Disease Affects Children With COVID-19
Find more cardiology related COVID-19 content
Reference:


 November 14, 2025
November 14, 2025 









