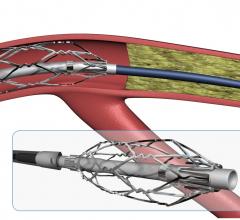
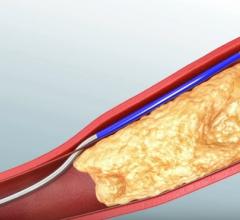
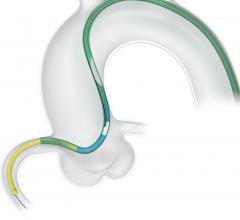
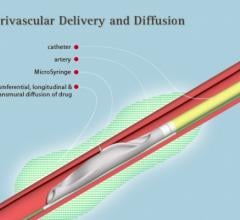
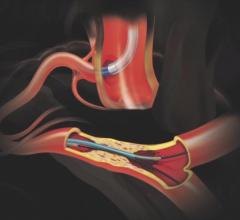
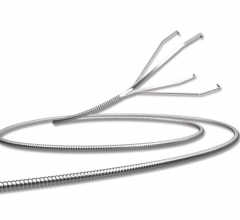
November 2, 2017 — Crossing the occlusion with a guidewire is often the most challenging part of chronic total occlusion ...
Catheters
October 5, 2017 — BTG plc announced it has acquired Roxwood Medical, provider of advanced cardiovascular specialty ...
October 4, 2017 — TVA Medical Inc. announced that its everlinQ 4 endoAVF System has received CE Mark in the European ...
October 2, 2017 — Reflow Medical Inc. announced that the company has received 510(k) clearance from the U.S. Food and ...
August 9, 2017 — Roxwood Medical Inc. recently announced it has entered into an exclusive agreement with Abbott for ...
June 28, 2017 — Royal Philips recently announced the relaunch of its Pioneer Plus catheter, the first and only re-entry ...
June 22, 2017 — The U.S. Food and Drug Administration (FDA) has identified Vascular Solutions’ recent recall of its ...
March 2, 2017 — Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S ...
February 17, 2017 — Medtronic plc announced that its coronary portfolio will now include the DxTerity Diagnostic ...
February 15, 2017 — Mercator MedSystems announced that the national co-principal investigators of the company’s DANCE tr ...
A discussion with guidewire expert Dimitri Karmpaliotis, M.D., Ph.D., FACC, about the basics of interventional guidewire ...
November 9, 2016 — Merit Medical Systems Inc. announced that it has received 510(k) clearance for the SwiftNINJA ...
October 18, 2016 — Medtronic plc announced last week that it has notified customers of a voluntary recall of certain ...
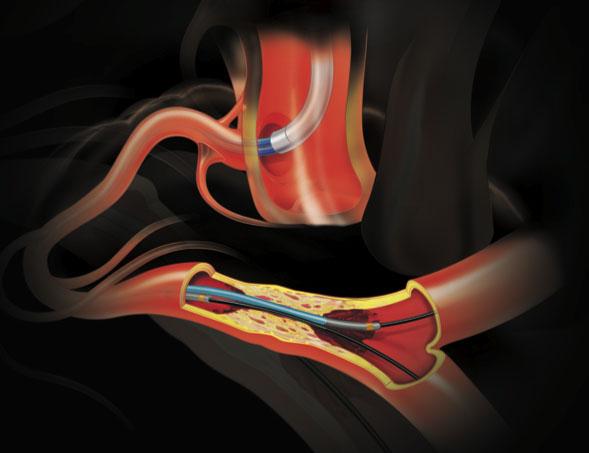
While guidewires are a key tool used by all interventionalists in the cath lab, most operators do not have a deep ...
October 12, 2016 — Medtronic plc announced recently that the U.S. Food and Drug Administration (FDA) has cleared the ...


 November 02, 2017
November 02, 2017