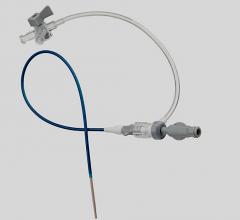
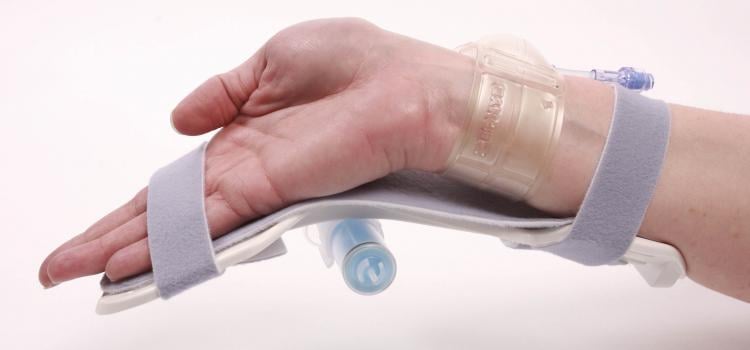
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform as both a guiding sheath and an introducer sheath, for use in peripheral ...
Vascular Access
This channel includes news and new technology innovations for vascular access, both venous and arterial access, for percutaneous, transcatheter, interventional devices. In cath lab procedures, an introducer sheath needs to be inserted into the vessel to allow access of guidewires, catheters and devices into the body. Introducer sheaths are self-sealing to serve a stop cock to prevent blood from leaving the body during procedures. There are various access sites that can be accessed, most commonly femoral or radial access, but also the ulnar, axillary and tibiopedal vasciular access routes There are several complications that can occur at the access site and technologies have been developed to reduce these issues, especially with the most common bleeding complications.
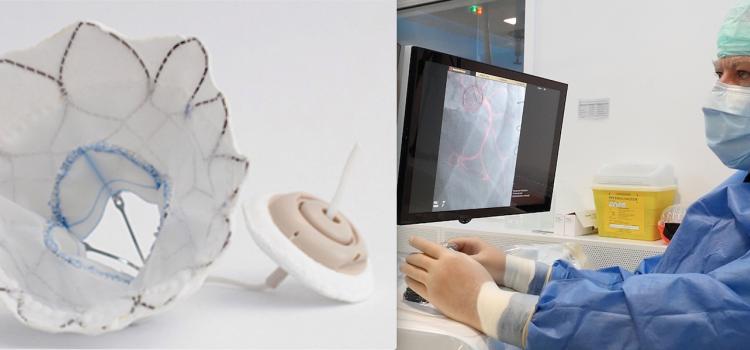
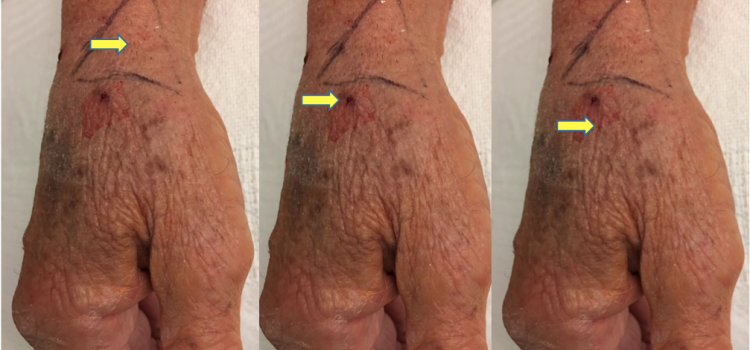
March 24, 2023 — The American College of Surgeons (ACS), with the Society for Vascular Surgery (SVS), has launched a new ...
November 2, 2022 — A number of awards of distinction were presented during The VEINS (Venous Endovascular INterventional ...
October 31, 2022 — Results from the Late-Breaking Clinical Trials session at The VEINS Conference in Las Vegas, NV, were ...
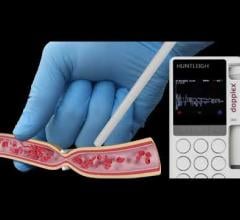
June 2, 2022 — With over 30 years' experience in vascular assessment, Huntleigh, a member of the Arjo family, has ...
May 6, 2021 - Bluegrass Vascular Technologies announced the publication of a report documenting the clinical utility of ...
February 8, 2021 — Teleflex Inc. said it recently completed its acquisition of Z-Medica LLC, an industry-leading ...
August 25, 2020 – Atlantic Health System’s Morristown Medical Center has opened one of the region’s first radial lounges ...

Vascular access site bleeding is associated with higher complications and mortality rates. For decades femoral access ...
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform ...
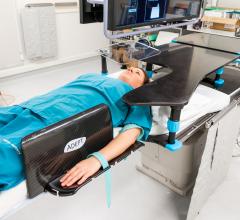
Transradial access for percutaneous coronary intervention (PCI) in the United States has grown rapidly over the past 20 ...
February 3, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) m ...
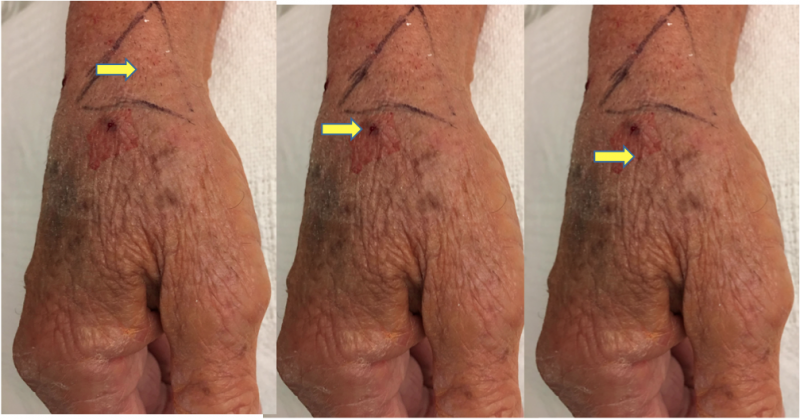
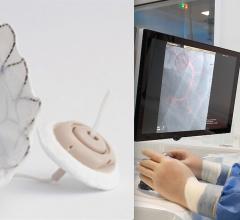
November 6, 2019 — Saranas Inc. announced completion of the first U.S. commercial case using its Early Bird Bleed ...
Sunil Rao, M.D., chief of cardiology, Durham VA Health System and a professor at Duke University, and Prashant Kaul, M.D ...
September 3, 2019 — New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically driven ...



 March 24, 2023
March 24, 2023