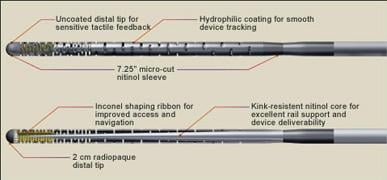
October 13, 2010 – A new guidewire has been launched for use in challenging, small vessel peripheral angioplasty procedures. The Journey Guidewire, by Boston Scientific, is 0.014 inches and designed to address physician needs in treating arteries below the knee.
“In my experience, the Journey Guidewire provides exceptional torque and flexibility, particularly in difficult cases involving small vessels or tortuous anatomy,” said James Benenati, M.D., medical director of the Baptist Cardiac and Vascular Institute in Miami, and president of the Society of Interventional Radiology. “This highly durable guidewire offers outstanding precision and tracking to help access the toughest peripheral lesions.”
Benenati used the Journey Guidewire in a live case demonstration at the Transcatheter Cardiovascular Therapeutics (TCT) 2010 symposium in Washington, D.C.
It features a micro-cut nitinol sleeve designed to provide efficient transmission of torque energy for more precise turn-by-turn response and control compared to conventional spring-coil guidewires. The nitinol distal core and hydrophilic coating are designed to enhance wire durability, tactile response and device delivery for improved overall performance.
For more information: www.bostonscientific.com


 February 12, 2026
February 12, 2026 









