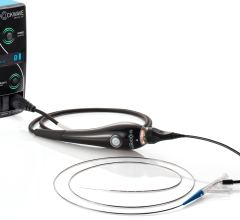
July 30, 2021 – Innovative Health Inc. announced several new initiatives to increase savings from single-use device reprocessing in the electrophysiology (EP) lab. The company has formed an emerging technologies team, charged with developing new reprocessing technologies that will enable cost-savings to be realized on more devices, modalities and clinical areas. Simultaneously, the company has launched a clinical integration team that will be working directly with EP labs to align clinical, technological, and administrative goals in order to simultaneously optimize savings and patient care quality.
The company made the announcement earlier July in preparation for the Heart Rhythm 2021 Scientific Sessions, the annual meeting of the Heart Rhythm Society (HRS).
“Single-use device reprocessing has been successfully proven to generate substantial savings for participating cardiology programs,” said Innovative Health CEO Rick Ferreira. “However, we have barely scratched the surface in terms of savings. To realize higher savings, we need to continue to develop our technology and work with our partners to ensure savings are maximized. This is an important investment in the future of reprocessing.”
The announcement comes as Innovative Health is on the verge of adding several new devices to its list of reprocessing opportunities. Four submissions are under review with U.S. Food and Drug Administration (FDA), some of which are for devices that represent the newest and most advanced technology within their modalities. Such clearances will enable labs to increase their per-procedure savings by hundreds of dollars.
Innovative Health’s investment in its newly formed emerging technologies team will also support its expansion into targeting savings opportunities outside of the EP lab. Annual savings potential in the cardiac cath lab (interventional cardiology) via reprocessing is estimated to be hundreds of millions of dollars a year. The vendor said similar to the savings that can be achieved in the EP lab. The company is expecting its first cath lab device clearance within the next few months.
Savings per procedure from reprocessing basic catheters are less than $1,000 per procedure, while savings on some diagnostic ultrasound and mapping catheters are more than $1,000 per device. Upgrading a basic EP reprocessing program to an advanced reprocessing program typically adds savings of more than $2,000 per procedure, or 100s of thousands of dollars per year, the company said.
“Innovative Health has enabled EP labs to reduce their costs considerably through single-use device reprocessing,” said Aaron DeTate, senior director of clinical integration at Innovative Health. “We are excited to take these savings across the hall to the cath lab and offer similar financial benefits to our colleagues in interventional cardiology.”
For more information: innovative-health.com


 December 20, 2023
December 20, 2023