Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) procedure, a minimally invasive treatment now available at Long Beach Medical ...
Catheter
This channel includes news and new technology innovations about catheters. Catheters are thin tubes interted into the body to remove fluid, treat diseases or perform surgical procedures.
Feb. 12, 2026 – CellProthera has acquired the transendocardial catheter originally developed by Celyad Oncology. This ...
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
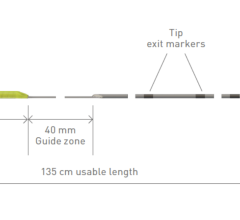
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) ...
Dec. 3, 2025 — Recross Cardio Inc., a structural heart company developing next-generation membrane sealing technology ...
Oct. 7, 2025 — Cagent Vascular has announced the commercial launch of the Serranator PTA Serration Balloon Catheter in 7 ...
Oct. 2, 2025 — AorticLab recently announced the U.S. Food and Drug Administration (FDA) has approved its Investigational ...
Sept. 29, 2025 — Radiation therapy may offer a comparable and potentially safer alternative to repeat catheter ablation ...
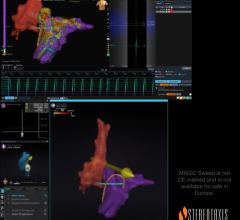
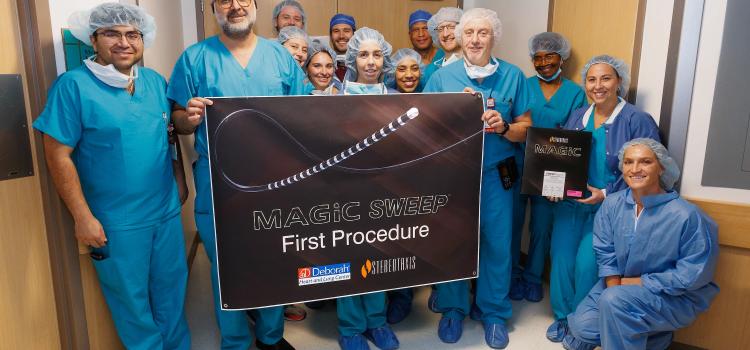
Sept. 2, 2025 — Stereotaxis has announced the successful completion of the world’s first procedures using MAGiC Sweep, a ...
July 28, 2025 — Stereotaxis, a provider of surgical robotics for minimally invasive endovascular intervention, has ...
Sept. 16, 2024 — Biotronik has received U.S. Food and Drug Administration (FDA) labeling approval of its Selectra 3D ...
June 7, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of ...
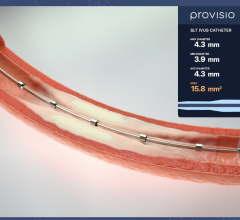
April 25, 2024 — Provisio Medical announced FDA 510(k) clearance of the Provisio SLT IVUS System. Sonic Lumen Tomography ...
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using Edward ...
February 9, 2024 — BIOTRONIK introduced the Micro Rx catheter, a novel rapid exchange microcatheter developed to easily ...




 February 12, 2026
February 12, 2026