According to a new report from Allied Market Research, the global catheters market was valued at $22.7 billion in 2021 ...
Catheter
This channel includes news and new technology innovations about catheters. Catheters are thin tubes interted into the body to remove fluid, treat diseases or perform surgical procedures.
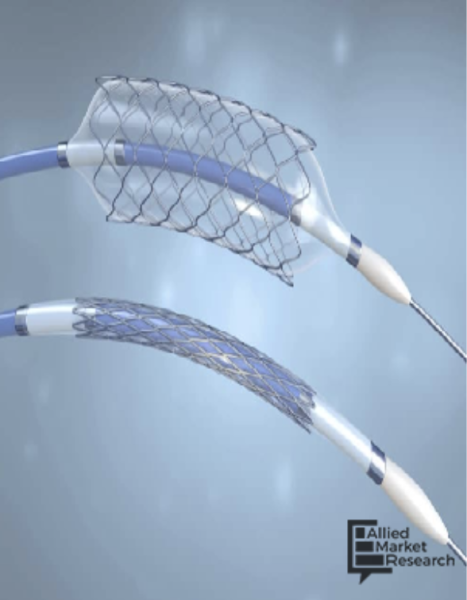
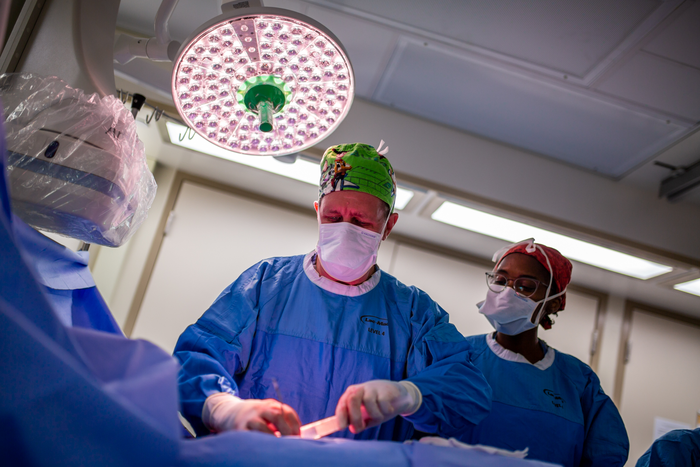
Here is what you and your colleagues found to be most interesting in the field of cardiology during the month of May ...
February 25, 2022 – Teleflex Incorporated, a leading global provider of medical technologies, today announced that the U ...

In the electrophysiology (EP) lab, hundreds of thousands of used devices are sent to reprocessors every year to get ...
July 30, 2021 – Innovative Health Inc. announced several new initiatives to increase savings from single-use device ...
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform ...
April 7, 2020 — Boston Scientific Corp. is recalling its Imager II 5 French angiographic catheters, because there is a ...
December 18, 2019 — Cook Medical initiated a recall of its CrossCath Support Catheters in November, which the U.S. Food ...
October 31, 2019 — Baylis Medical announced the first North American use of its EPstar Fixed Electrophysiology Catheters ...
October 9, 2019 — Medtronic is recalling the 6 French Sherpa NX Active Guide Catheter due to a risk of the outer ...
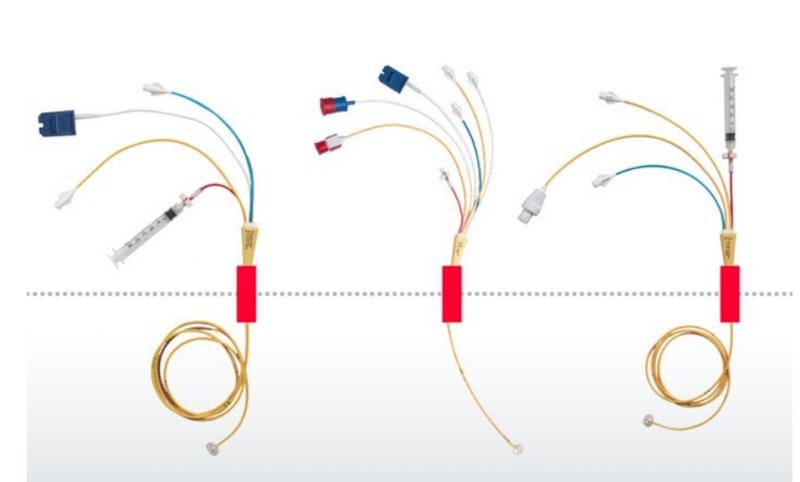
Edwards Lifesciences is recalling its 131F7, 131F7J, 131F7P, 131VF7P, 151F7 Swan-Ganz Thermodilution Catheters ...
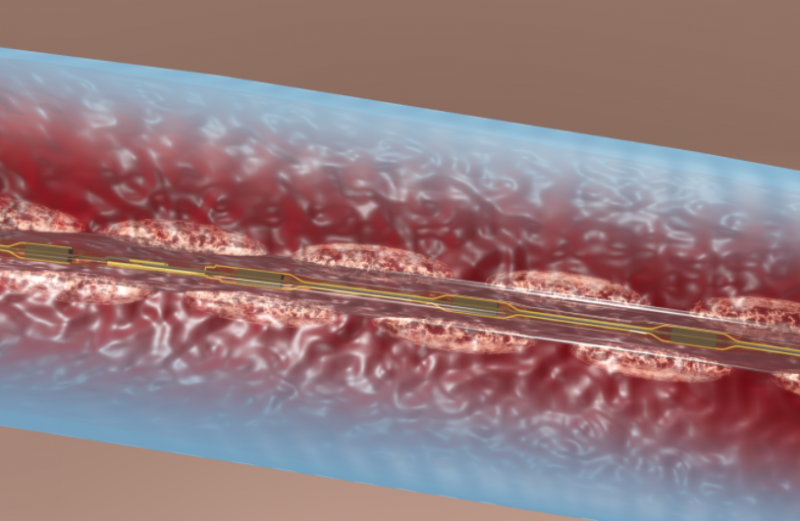
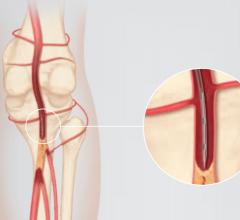
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
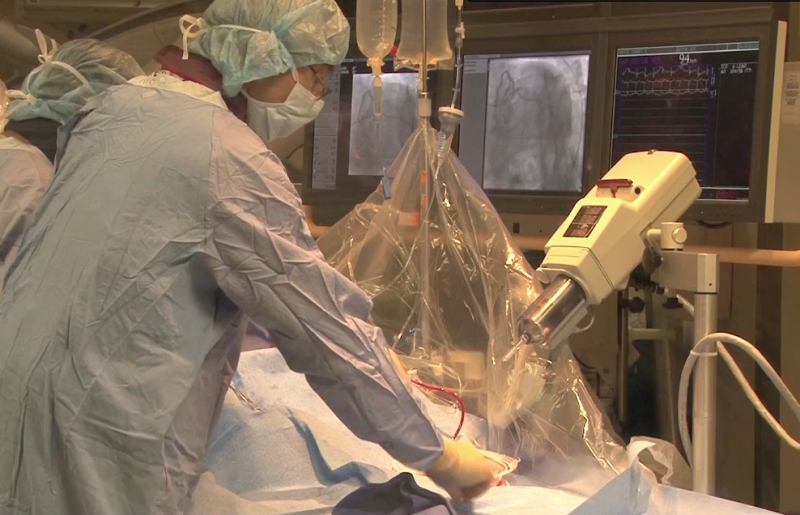
As payers and other healthcare entities look to better manage costs, especially in the acute care setting, it is ...
June 22, 2017 — The U.S. Food and Drug Administration (FDA) has identified Vascular Solutions’ recent recall of its ...

 June 09, 2022
June 09, 2022