July 24, 2025 — Royal Philips has announced a collaboration with Epic to integrate Philips’ suite of cardiac ambulatory ...
July 22, 2025 — In a collaboration between American Heart Association Ventures and the Association’s Center for Health ...
July 21, 2025 — Long COVID patients with abnormal cardiopulmonary PET/MR findings may be more likely to develop heart ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 16, 2025 — Medtronic has announced that the first patient has been enrolled in the PEripheral Onyx Liquid Embolic ...
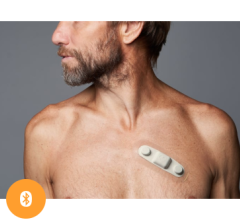
July 22, 2025 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care by providing powerful ...
July 21, 2025. — Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David's Medical Center ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 17, 2025— Heartflow, Inc. recently announced new data for its AI-enabled Heartflow Plaque Analysis, including final ...
July 17, 2025 — Royal Philips, a global leader in health technology, has launched the Philips ECG AI Marketplace, a ...
July 15, 2025 – Cleerly has announced that EviCore, a leading radiology benefit manager providing coverage guidelines to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 16, 2025 — The American Heart Association has appointed two leading health and science leaders, Jennifer Ashton, M ...
July 11, 2025 — Heartflow, Inc. has announced that its Heartflow Plaque Analysis will be included in updated cardiac ...
July 9, 2025 — Cleerly, a leader in advanced cardiovascular imaging, will be presenting new research at the Society of ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 10, 2025 — Elucid, an AI medical technology company focused on providing physicians with a more precise view of ...
July 15, 2025 — Vivalink, a provider of digital healthcare solutions, has announced its wearable ECG monitor is ...
July 14, 2025 – Johnson & Johnson MedTech has announced U.S. Food and Drug Administration (FDA) approval of an update ...


 July 24, 2025
July 24, 2025