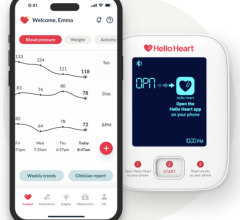
April 24, 2024 —Hello Heart, a digital leader in preventive heart health, today announced results from its latest study observing the benefits of scalable, accessible digital health tools for a ...
Did you know that you have instant access to the latest webinars from Diagnostic and Interventional Cardiology? DAIC's ...
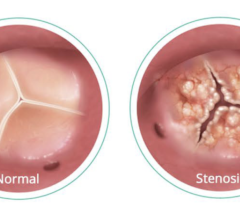
April 25, 2024 —Heart-Valve-Surgery.com, a leading patient advocacy group for heart valve disease, with support from ...
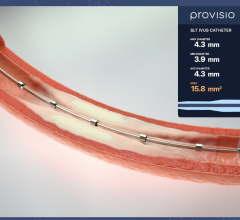
April 25, 2024 — Provisio Medical announced FDA 510(k) clearance of the Provisio SLT IVUS System. Sonic Lumen Tomography ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using ...
April 24, 2024 — Heart Test Laboratories, Inc. d/b/a HeartSciences, an artificial intelligence (AI)-powered medical ...
April 24, 2024 — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
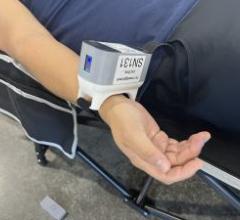
April 24, 2024 —Hello Heart, a digital leader in preventive heart health, today announced results from its latest study ...
April 23, 2024 — CDL Nuclear Technologies, a pioneer in advanced diagnostic solutions, is proud to announce the launch ...
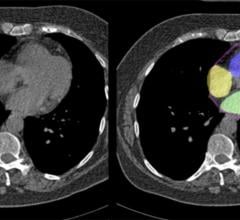
April 23, 2024 — A recent study designed and implemented by investigators at Cedars-Sinai found that artificial ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
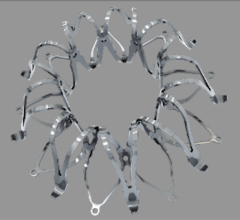
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...
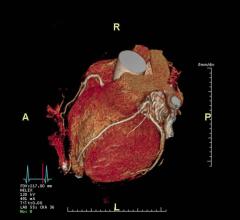
April 22, 2024 — A new study showed that a non-invasive imaging test can help identify patients with coronary artery ...
April 22, 2024 — At the annual American College of Cardiology conference (ACC.24) in Atlanta last week, RCE Technologies ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
April 22, 2024 — Corvia Medical, Inc, a company dedicated to transforming the treatment of heart failure, welcomes the ...
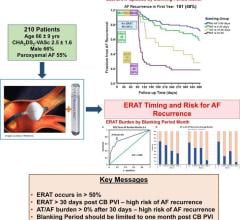
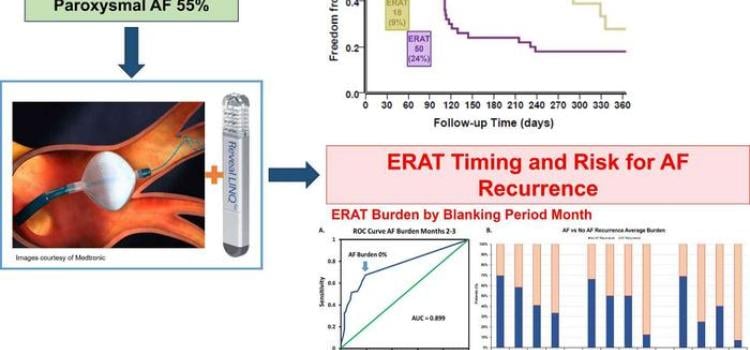
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period ...
April 18, 2024 — Karoo Health, the only operational provider of cardiac value-based care (VBC) enablement with published ...




 April 26, 2024
April 26, 2024