February 8, 2017 — Cardiovascular Systems (CSI) presented six-month data from its LIBERTY 360° post-market study in a ...
A new anticancer agent in development promotes regeneration of damaged heart muscle — an unexpected research finding that may help prevent congestive heart failure in the future.
GE Healthcare’s Life Sciences business announced in January that it acquired Rapidscan Pharma Solutions Inc., which has the exclusive rights to produce and sell the pharmacological stress agent Rapiscan (regadenoson) in territories outside the U.S., Canada and Mexico. GE Healthcare will help bring improved access to Rapiscan, offering an alternative screening method for patients who are unable to undergo traditional cardiac stress imaging procedures.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Healthcare analytics company Innovaccer Inc. announced the launch of its holistic MIPS Platform designed to enable providers to deliver better clinical outcomes. The platform does this by helping providers monitor performances, understand population, efficiently manage data and easily submit it to the Centers for Medicare and Medicaid Services (CMS).
February 7, 2017 — Of the more than 700,000 Americans who suffer a heart attack each year, about a quarter go on to ...
The supply chain can serve as a critical strategic asset when addressing important initiatives tied to managing costs ...
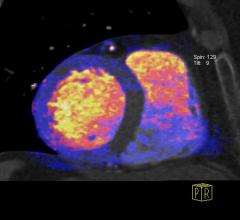
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
At the 2016 Radiological Society of North America (RSNA) annual meeting, GE Healthcare unveiled Freelium, a magnet technology designed to use 1 percent of liquid helium compared to conventional magnetic resonance imaging (MRI) magnets. Instead of the average 2,000 liters of precious liquid helium, Freelium is designed to use only about 20 liters.
February 6, 2017 — At the Society of Cardiac Magnetic Resonance (SCMR) 20th Annual Scientific Sessions, GE Healthcare ...
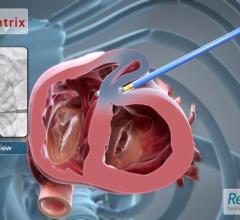
This video, provided by BioVentrix Inc., demonstrates how to implant the Revivent TC System to reduce the volume of the ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Avinger Inc. recently announced positive two-year clinical data from the pivotal VISION study of the company’s Lumivascular technology. The VISION study was designed to evaluate the safety and effectiveness of Avinger’s Pantheris system to perform directional atherectomy while, for the first time ever, allowing physicians to use real-time intravascular imaging to aid in the removal of plaque from diseased lower extremity arteries. Data from the study, which demonstrated successful achievement of all primary and secondary safety and effectiveness endpoints, supported U.S. Food and Drug Administration (FDA) 510(k) clearance of the system in 2016.
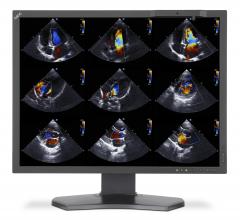
For over 20 years, stress echocardiography (SE) has been widely used to help clinicians diagnose ischemic heart disease, in which coronary arteries have narrowed, leading to restricted blood flow and damage to the heart. However, in recent years, stress echocardiography has also become an established method to assess a much wider array of complex heart conditions, such as heart failure and valvular heart disease. A new document, The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE), aims to establish guidance for how best to apply, perform and interpret SE for patients with a multitude of conditions.
Penn Medicine announced that its heart failure team is using big data to kick-start a project that is working to improve communication across the continuum of care, and ultimately reduce readmissions for heart failure patients.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Physicians and researchers at Texas Heart Institute are recruiting patients who suffer from heart failure to participate in the first-ever clinical study of combination adult stem cell therapy in cardiovascular medicine. Adult cell therapy has been studied in patients with heart disease with an excellent safety profile, though a combination of two cell types has not yet been evaluated in cardiovascular disease.
Lantheus Medical Imaging Inc. announced U.S. Food and Drug Administration (FDA) approval of a label update for Definity Vial for (Perflutren Lipid Microsphere) Injectable Suspension. The update removes the contraindication statement related to use in patients with a known or suspected cardiac shunt from the U.S. Prescribing Information.
Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labeling for both the Assurity MRI pacemaker and the Tendril MRI pacing lead. Patients implanted with these low-voltage devices will have the ability to undergo full body magnetic resonance imaging (MRI) scans, if required. With the approval, the Assurity MRI pacemaker is now the world's smallest, longest-lasting wireless MRI-compatible pacemaker, according to Abbott.


 February 08, 2017
February 08, 2017