
September 26, 2012 — Abbott Vascular this week began its international launch of the Absorb coronary stent, the world's first drug-eluting bioresorbable vascular scaffold (BVS). It is now widely available across Europe and parts of Asia Pacific and Latin America. The Absorb is a first-of-its-kind device for the treatment of coronary artery disease (CAD). It works by restoring blood flow to the heart similar to a metallic stent, but then dissolves into the body, leaving behind a treated vessel that may resume more natural function and movement because it is free of a permanent metallic stent. Absorb is made of polylactide, a naturally dissolvable material that is commonly used in medical implants such as dissolving sutures.
Clinical data shows there are significant potential long-term benefits of a scaffold that dissolves. After it dissolves, the vessel can expand and contract as needed to increase the flow of blood to the heart in response to normal activities such as exercising. In addition, future treatment and diagnostic options are broadened. The stent also reduces the need for long-term treatment with antiplatelet medications. Future interventions also would be unobstructed by a permanent implant.
"This innovation represents a true paradigm shift in how we treat coronary artery disease. With the launch of Absorb, a scaffold that disappears after doing its job is no longer a dream, but a reality," said Patrick W. Serruys, M.D., Ph.D., professor of interventional cardiology at the Thoraxcentre, Erasmus University Hospital, Rotterdam, the Netherlands. "Patients are excited about Absorb since it may allow blood vessels to return to a more natural state and expand long-term diagnostic and treatment options."
The international launch of Absorb is supported by a robust clinical trial program that encompasses five studies in more than 20 countries around the world. Study data indicate that Absorb performs similar to a best-in-class drug eluting stent across traditional measures such as major adverse cardiovascular events (MACE) and target lesion revascularization (TLR), while providing patients with the added benefits associated with a device that dissolves over time. As the Absorb scaffold dissolves, vascular function is potentially restored to the blood vessel, allowing more blood to flow through the vessel as the body requires.
"Absorb is a leading example of Abbott's dedication to advancing patient outcomes through innovative technology. Abbott has remained committed to meeting the growing physician and patient demand for a bioresorbable vascular scaffold – from the initial device developed nearly 10 years ago to the expansion of our manufacturing capabilities to support this international launch," said John M. Capek, executive vice president, Medical Devices, Abbott. "We are proud to be the first company to commercialize a drug-eluting bioresorbable vascular scaffold, which has the potential to revolutionize the way physicians treat their patients with coronary artery disease."
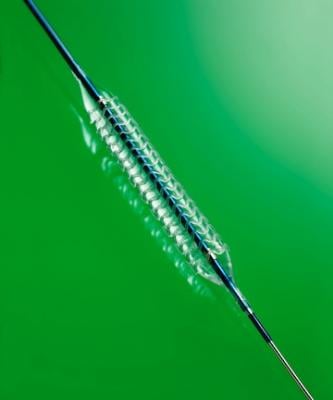
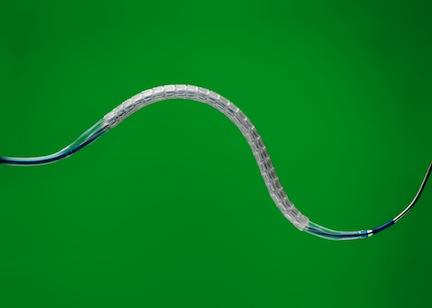
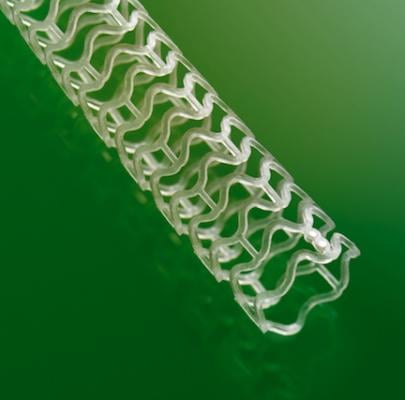
The Absorb Bioresorbable Vascular Scaffold
Absorb is now available in a broad size matrix to support the needs of physicians treating patients with CAD.
The Absorb bioresorbable vascular scaffold, similar to a small mesh tube, is designed to open a blocked heart vessel and restore blood flow to the heart. Absorb is referred to as a scaffold to indicate that it is a temporary structure, unlike a stent, which is a permanent implant. The scaffold provides support to the vessel until the artery can stay open on its own, and then dissolves naturally. Absorb leaves patients with a vessel free of a permanent metallic stent and may allow the vessel to resume more natural function and movement, enabling long-term benefits.
Abbott's BVS delivers everolimus, an antiproliferative drug used in Abbott's Xience coronary stent systems. Everolimus was developed by Novartis Pharma AG and is licensed to Abbott by Novartis for use on its drug-eluting vascular devices. Everolimus has been shown to inhibit in-stent neointimal growth in the coronary vessels following stent implantation, due to its antiproliferative properties.
Absorb is neither approved nor authorized for sale and currently is in development with no regulatory status in the United States. Absorb is authorized for sale in CE mark countries. Absorb is now available in Europe, the Middle East, parts of Asia Pacific (including Hong Kong, Singapore, Malaysia and New Zealand) and parts of Latin America.
For more information: www.abbott.com


 November 24, 2025
November 24, 2025 









