March 31, 2014 — Led by members of the American College of Cardiology (ACC), American Heart Association (AHA) and Society for Cardiovascular Angiography and Interventions (SCAI), 14 professional societies from across the world have developed a health policy statement that defines the clinical standards for structured reporting in the cardiac catheterization suite. The statement was published online last week in the Journal of the American College of Cardiology and Circulation.
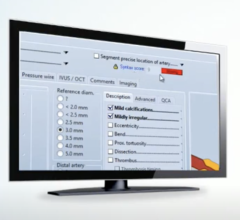
The goal of structured reporting is to produce clear, concise, thorough and organized reports on catheterization procedures that include all information relevant to both clinical care and operational administration. For instance, it should include a minimum dataset that anticipates clinical, operational, regulatory and financial uses, and documents indications and appropriateness.
The result of this approach should be data-intensive reports that concisely and efficiently convey the details of a variety of catheterization procedures, such as those involving the heart and carotid arteries, peripheral blood vessels, pediatric and adult congenital and structural heart conditions, and valve replacements. The clarity, consistency and quality of these procedure reports will be a marked improvement over current approaches, which are inconsistent and vary widely among catheterization labs and vendors.
“Getting everyone on the same page is crucial to improving care for the patient,” said Tim Sanborn, M.D., MS, FACC, FAHA, FSCAI, clinical professor of medicine, University of Chicago Pritzker School of Medicine, and project chair. “Structured reporting increases the efficiency and effectiveness of the entire catheterization team, improves communication and coordination of care, and reduces delays in care. In addition, it facilitates the use of data for clinical care, quality assessment, performance improvement, billing, regulatory and other purposes.”
The new published statement offers specific report templates to illustrate the principles of structured reporting. These templates help users manage the patient’s data before, during and after the procedure and integrate data management into the workflows of all care team members. They provide a step-by-step process from scheduling to analysis and outline how to compile a report once the procedure is complete. They also identify when, where and how the processes of data are integrated into the workflow.
“These data-intense reports will efficiently convey the details of the procedure, findings, analyses and recommendations of care for the patient,” said James E. Tcheng, M.D., FACC, FSCAI, FESC, interventional cardiologist, director of Duke Information Systems for cardiovascular care, Duke University Health System, Durham, N.C., and vice chair of the project. “They will reduce the documentation burden and allow physicians to focus more on care recommendations for the patient.”
Widespread adoption of this structured reporting approach will require a substantial transformation of catheterization laboratory reporting that will affect administration, physicians and staff. This transformation, however, is aligned with federal initiatives promoting the universal adoption of electronic health records (EHRs).
In providing report templates, the joint effort will help accelerate the development and application of this new approach to reporting catheterization procedures. Yet also critical to the success of this effort is for health information technology vendors to build and implement systems that enable structured reporting.
For more information: www.cardiosource.org, www.americanheart.org, www.scai.org, www.hl7.org, www.ihe.net, www.sts.org and www.vascularweb.org


 November 06, 2025
November 06, 2025